Electrochemistry
Voltaic cell :
Charge ( Q ) = Current ( I ) × time ( t )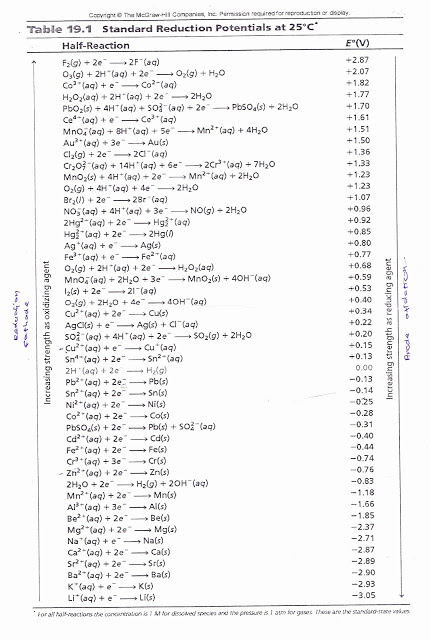
- 10.2 Nernst Equation
Electrochemistry : 10.2 Nernst Equation NERNST EQUATION Cell potential (E°cell) under any condition Ecell = E°cell – (RT/nF) ln Q R: universal gas constant. Q...
- Standard Hydrogen Potential
Standard Reduction PotentialsStandard reduction potential (E0) is the voltage associated with a reduction reaction at an electrode when all solutes are 1 M and all gases are at 1 atm. Standard hydrogen electrode (SHE) Reduction Reaction ...
- Galvanic Cell
Oxidation is defined as the addition of oxygen to a substancethe loss of hydrogen from a substancethe...
- Electrochemistry : 10.2 Nernst Equation
NERNST EQUATION Cell potential (E°cell) under any condition Ecell = E°cell – (RT/nF) ln Q R: universal gas constant. Q...
- Thermochemistry : 9.3 Born-haber Cycle
First Ionization Energy (IE1)Energy required for 1 mol of gaseous atom to lose 1 mol of electrons. Affinity Electron (EA) Energy change that occurs when 1 mol of gaseous atom gains 1 mol of electrons. Lattice EnergyEnergy change when 1 mol of solid...
Electrochemistry
Electrochemistry : 10.3 : Electrolysis cell
VOLTAN CELL VS ELECTRIOLYSIS CELL .
Voltaic cell :
- use a spontaneous reaction to generate electric energy.
Electrolysis :
- use electric energy to drive non- spontaneous energy.
VOLTAIC CELL. | ELECTROLYTIC |
|
|
|
|
|
|
ELECTROLYSIS
- Splitting ('lysing') of a substance by input of electrical energy.
- To decompose a compound into it element.
ELECTROLITE IN AN ELECTROLYTIC CELL.
1. Can be :
- pure compound.
ex : H2O , molten salt
- Aqueous solution of salt.
ex : NaCI(aq) Na2SO4 (aq)
ELETROLYSIS OF PURE MOLTEN SALT
Example : molten NACI
Anode (oxidation) : 2Cl (l) ------ Cl2(g) + 2e-
Cathode (reduction) : 2Na+ (l) + 2e- ------ 2Na (s)
Overall : 2Na+ (l) + 2Cl (l) ------ 2Na + Cl2 (g)
*anion oxidised at anode!
*cation reduced at cathode!
ELECRTROLYSIS OF WATER
Anode (oxidation) 2H2O(l) ------ O2(g) + 4H+(aq) + 4e-
Cathode (reduction) 4H2O(l)+ 4e- ------ 2H2(g) + 4OH-(aq)
Overall 2H2O(l) ------ 2H2(g) + O2(g)
[Note : 4H+(aq) + 4OH-(aq) ------ 4H2O(l)]
- volume of H2 : O
= 2 : 1
- electrode : inert (Pt, etc )
- H2O oxidized at cathode produce O2
- H2O reduce at cathode to produce H2
- water can be oxidized and reduced.
ELECTROLYSIS OF AQUEOUS IONIC SOLUTION
- Aqueous solution of salt are mixture of many species (ions and HO2)
- So we have to compare various electrode potentials (E◦) to predict.
PREDICTING ELECTROLYSIS PRODUCT
- When two half- reaction are possible at an electrode.
Cathode : reduction with more positive E° occurs.
Anode : oxidation with more negative E° occurs.
CATION OF ACTIVE METALS (that cannot be reduced)
- Cations of metal in group (1) and (2) and A 1
- They are not reduced (E° more negative)
- H2O reduced to H2 and OH
Example: Na2SO4(aq)
Species present in solution: Na+ , SO42-, H2O
At cathode(-):
Na+(aq) + e- ------ Na(s) E= -2.71 V
2H2O(l) + 2e- ------ H2(g) + 2OH- (aq) E= -0.83 V
ANION (OXOANIONS AND F- ) (cannot be oxidised)
- Common oxoanion such as SO42-, CO32-, HO3-, AND PO43-, (AND F) are not oxidized.
- Because the central atom already at it highest oxidation state.
- H2O oxidixed to O2 and H+
EXAMPLE :Na2SO4(aq)
Special present in the solution: Na+, SO42-, H2O
At anode(+):
2H2O ------ O(g) + 4H+(aq) + 4e-
CATION OF LESS ACTIVE METALS (can be reduce)
- Cation of Au, Ag, Cu, Cr, Pb and Cd
- They are reduce at cathode (E° more +ve)
Example: AgNO(aq)
Species present in the solution: Ag+ , NO-, H2O
At cathode(-):
Ag+(aq) + e- ------ Ag(s)
2H2O(l) + 2e- ------ H(g) + 2OH-(aq)
HALIDES THAT CAN BE OXIDIZER
- I-, Br-, Cl-, except F
- The concentration must be high
Example : NaCl(aq)
Species present in the solution : Na+, Cl-, H2O
At cathode(-):
2H2O(l) + 2e- ------ H2(g) + 2OH-(aq)
At anode(+):
2Cl-(aq) ------ Cl2(g) + 2e-
SUMMARY ON PREDICTING ELECTROLYSIS PRODUCT
- Which species will be reduced : Au3+(aq) or H2O
ans : Au3+
Au3+(aq) + e- ------ Au(s)
Au3+ is ion of less active metal (E more positive).
- Which species will be reduces : NA+(aq) or H2O
ans : H2O
2H2O(l) + 2e- ------ H2(g) + 2OH-(aq)
Na+ is ion of active metal (E more negative).
- Which species will be oxidised:
ans : H2O
2H2O(l) + O(g) ------ 4H+(aq) + 4e-
SO42- is an oxoanion (cannot be oxidised because the central atom alreary at the highest oxidation states).
- Which species will be oxidized: CL (aq) or H2o
ans : Cl
2Cl-(aq) ------ Cl2(g) + 2e-
hallides (except F-) can be oxidized (due to overvoltage).
EFFECT OF CONCENTRATION
Ex: Concentration NaCl(aq)
At cathode (-) : 2H2O(l) + 2e- ------ H2(g) + 2OH-(aq)
At anode (+) : 2Cl-(aq) ------ Cl2(g) + 2e-
EXAMPLE : dilute NaCl(aq)
At cathode (-) 4H2O(l) + 4e- ------ 2H2(g) + 4OH-(aq)
At anode (+) 2H2O(l) ------ O2(g) + 4H+(aq) + 4e-
Overall 2H2O(l) ------ 2H2(g) + O2(g)
TYPE OF ELECTRODE
- -They do not involve in the reactionInactive electrode such as graphite and Pt(s) are normally use in electrolysis.
- EXAMPLE : Electrolysis of using inert electrodeActive electrodes such as metal (anode) dissolve to form metallic ions
- Cathode : Cu2+(aq) + 4e- ------ 2Cu (s)
- Anode : 2H2O(l) ------ O2(g) + 4 H+ (aq) + 4e-
- Overall : 2H2O(l) + 2Cu2+(aq) ------ 2Cu(S) + O2(g) + 4 H+ (aq)
FARADAY LAW
- Amount of substance produce of each electrode is directly proportional to quantity of charge flowing through the cell
- Also called Faraday's First Law of electrolysis.
CALCULATING USING FARADAY'S LAW
- Main steps
- Balance half reaction to find number of moles of electrons.
- Needed per mole product.
- Use Faraday's constant (96500C / mol ) to find corresponding charge.
- Use molar mass / mole to find charge needed for a given mass / mole of product
ELECTRIC CHARGE (Q)
Charge ( Q ) = Current ( I ) × time ( t )
Unit Coulomb , C Ampere , A Second , s

- 10.2 Nernst Equation
Electrochemistry : 10.2 Nernst Equation NERNST EQUATION Cell potential (E°cell) under any condition Ecell = E°cell – (RT/nF) ln Q R: universal gas constant. Q...
- Standard Hydrogen Potential
Standard Reduction PotentialsStandard reduction potential (E0) is the voltage associated with a reduction reaction at an electrode when all solutes are 1 M and all gases are at 1 atm. Standard hydrogen electrode (SHE) Reduction Reaction ...
- Galvanic Cell
Oxidation is defined as the addition of oxygen to a substancethe loss of hydrogen from a substancethe...
- Electrochemistry : 10.2 Nernst Equation
NERNST EQUATION Cell potential (E°cell) under any condition Ecell = E°cell – (RT/nF) ln Q R: universal gas constant. Q...
- Thermochemistry : 9.3 Born-haber Cycle
First Ionization Energy (IE1)Energy required for 1 mol of gaseous atom to lose 1 mol of electrons. Affinity Electron (EA) Energy change that occurs when 1 mol of gaseous atom gains 1 mol of electrons. Lattice EnergyEnergy change when 1 mol of solid...
