Electrochemistry

- 10.2 Nernst Equation
Electrochemistry : 10.2 Nernst Equation NERNST EQUATION Cell potential (E°cell) under any condition Ecell = E°cell – (RT/nF) ln Q R: universal gas constant. Q...
- Galvanic Cell
Oxidation is defined as the addition of oxygen to a substancethe loss of hydrogen from a substancethe...
- Electrochemistry : 10.2 Nernst Equation
NERNST EQUATION Cell potential (E°cell) under any condition Ecell = E°cell – (RT/nF) ln Q R: universal gas constant. Q...
- Electrochemistry : 10.1 Galvanic Cell (continued)
SPONTANEOUS REACTION occurs as the result of different ability of metal to give up their electron to flow through the circuit. CELL POTENTIAL (ECell) different in electrical potential of electrodes also called voltage or electromotive...
- Thermochemistry : 9.3 Born-haber Cycle
First Ionization Energy (IE1)Energy required for 1 mol of gaseous atom to lose 1 mol of electrons. Affinity Electron (EA) Energy change that occurs when 1 mol of gaseous atom gains 1 mol of electrons. Lattice EnergyEnergy change when 1 mol of solid...
Electrochemistry
Standard hydrogen potential
Standard Reduction Potentials
Standard reduction potential (E0) is the voltage associated with a reduction reaction at an electrode when all solutes are 1 M and all gases are at 1 atm.
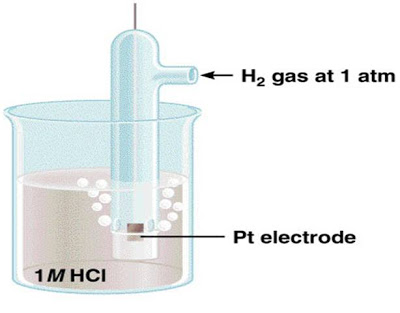 |
Standard hydrogen electrode (SHE) |
Reduction Reaction

E0 = 0 V
- 10.2 Nernst Equation
Electrochemistry : 10.2 Nernst Equation NERNST EQUATION Cell potential (E°cell) under any condition Ecell = E°cell – (RT/nF) ln Q R: universal gas constant. Q...
- Galvanic Cell
Oxidation is defined as the addition of oxygen to a substancethe loss of hydrogen from a substancethe...
- Electrochemistry : 10.2 Nernst Equation
NERNST EQUATION Cell potential (E°cell) under any condition Ecell = E°cell – (RT/nF) ln Q R: universal gas constant. Q...
- Electrochemistry : 10.1 Galvanic Cell (continued)
SPONTANEOUS REACTION occurs as the result of different ability of metal to give up their electron to flow through the circuit. CELL POTENTIAL (ECell) different in electrical potential of electrodes also called voltage or electromotive...
- Thermochemistry : 9.3 Born-haber Cycle
First Ionization Energy (IE1)Energy required for 1 mol of gaseous atom to lose 1 mol of electrons. Affinity Electron (EA) Energy change that occurs when 1 mol of gaseous atom gains 1 mol of electrons. Lattice EnergyEnergy change when 1 mol of solid...
