Electrochemistry
- Diversinate Update
Since our first report in 2011 on the trifluoromethylation of heterocycles, sulfinate chemistry has been demonstrated to be a useful tool for drug discovery. The lab extended this chemistry beyond trifluoromethyl radicals to a whole host of...
- Formal Olefin Hydroamination With Nitroarenes
Our latest work in the field of iron chemistry came out today in Science. First we would like to present a graph (shown below) on how this newly developed chemistry could simplify the synthesis of some biologically active intermediates: starting from...
- Reflecting On Yield, And The Need For A Qualitative Assessment Of Reactions
Here's some thoughts: 1. I categorize all reaction yields qualitatively. What does this mean? Generally speaking, I don't really believe in yields other than 0%, 25%, 50%, 75%, or quantitative. I also sometimes break this down as 1.)...
- A Taxol Synthesis Is Simple
I got the results back from NSF fellowship application today. I didn't get the fellowship, but that doesn't matter. Something funny was that one of the reviewers said my “proposed project looked simple or at least written...
- Column Chromatography....seems Silly Now.
Last month the team at Eisai responsible for the commercial scale synthesis of Halaven (easily the most complex drug molecule produced entirely by chemical synthesis) published a series of papers (theres three links there) detailing the development...
Electrochemistry
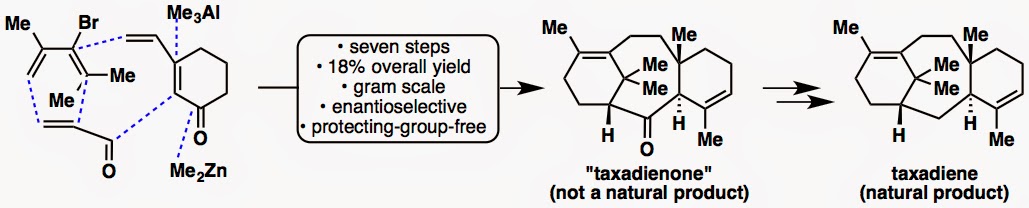
Academia–industry collaboration in the route optimization of taxadienone
In early 2012, we reported the gram-scale synthesis of a non-natural taxane, taxadienone, as well as that of a natural taxane, taxadiene. Now, chemists at Albany Molecular Research Inc. (AMRI) report a route optimization of taxadienone in none other than the process chemistry journal, OPRD.

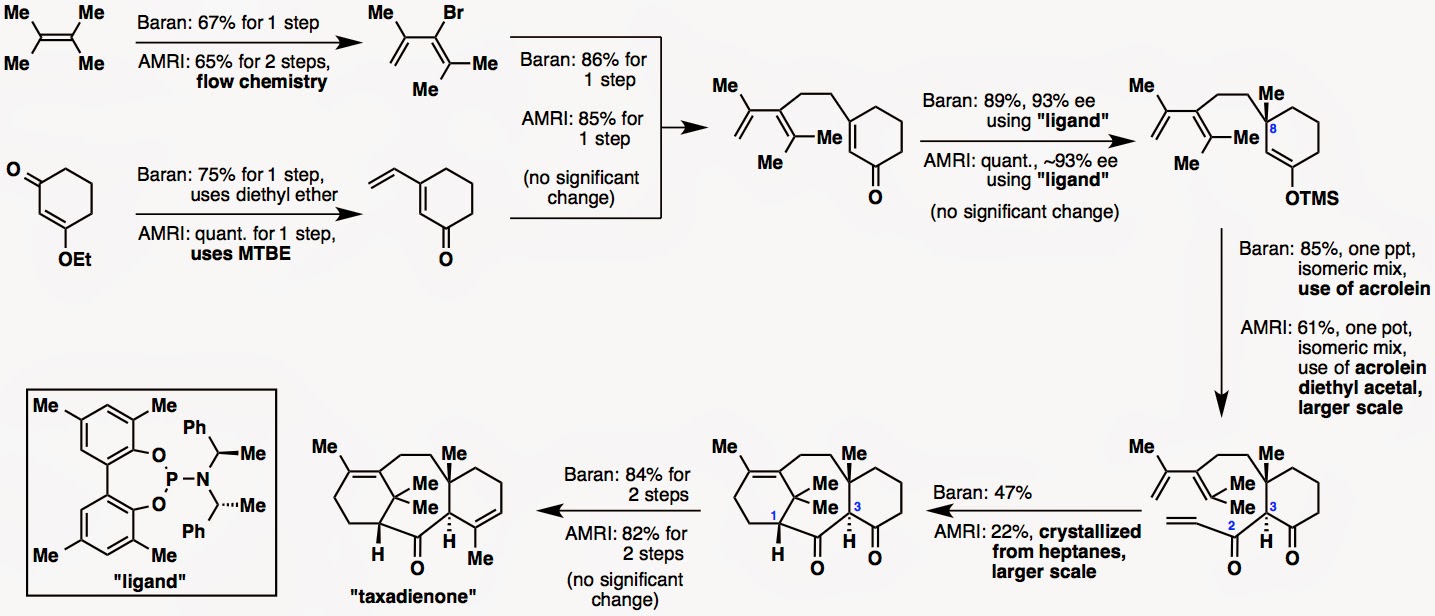
Before we describe how the story of this collaboration came about, we will take you through the route optimization process. The synthetic route itself is identical, as all the intermediates of our synthesis appear in their synthesis as well, but the reactions have been scaled up and the yields have been improved.
Reaction scale-up: We made ~2 g of taxadienone whereas AMRI made ~10 g.
Reaction yields: Please see the figure below.

Although the contents of AMRI’s paper will not be reiterated, we will show their concluding paragraph here (almost verbatim from their manuscript):
“The reported route to taxadienone was successfully optimized and scaled-up to decagram quantity. Thermal hazards associated with the production of bromodiene were addressed by employing a continuous flow reactor. The crystallization of a cyclized diketone at the penultimate step proved to be a decisive factor for obtaining taxadienone of high quality.”
Now, as for the behind-the-scenes story. This story started out as an interesting “experiment” in academia-industry collaboration. Our laboratory is engaged in many collaborations with industrial groups, including LEO Pharma, Bristol-Myers Squibb, and Sigma-Aldrich, and in most cases, our industrial partner has a project goal toward which we provide expertise and in-house research findings (industry —> academia outsourcing). This Baran–AMRI collaboration has actually been a “reverse collaboration” in which our initial synthetic route was taken up by an industrial group for scale-up (academia —> industry outsourcing). Through many interactions, by email, by phone and in person, AMRI saved our group much time and effort by generating large amounts of enantioenriched taxadienone. With this extra time in hand, we were able to study the front-line chemistry for longer periods of time, resulting, for example, in the synthesis of taxuyunnanine D. AMRI’s work also validated our synthesis by having an independent group reproduce our results, even when some of the reactions can be tricky. This “field-testing” of chemistry further refined our initial work, when some reactions were difficult to scale up (even though our initial synthesis was performed on a decent scale already).
Although this sort of “reverse” academia-industry collaboration is rare, we learned a lot from this experience! We understand that there is a time and place for this type of collaboration but we believe that in the near future, such collaborative work will be more commonplace. Finally, this is a wonderful advertisement for the impressive capabilities of the AMRI team and we recommend all our industrial friends that are looking to outsource challenging chemistry to give AMRI a try!
 |
| www.amriglobal.com |
Written by Yoshihiro Ishihara
Uploaded by Nathan Wilde
- Diversinate Update
Since our first report in 2011 on the trifluoromethylation of heterocycles, sulfinate chemistry has been demonstrated to be a useful tool for drug discovery. The lab extended this chemistry beyond trifluoromethyl radicals to a whole host of...
- Formal Olefin Hydroamination With Nitroarenes
Our latest work in the field of iron chemistry came out today in Science. First we would like to present a graph (shown below) on how this newly developed chemistry could simplify the synthesis of some biologically active intermediates: starting from...
- Reflecting On Yield, And The Need For A Qualitative Assessment Of Reactions
Here's some thoughts: 1. I categorize all reaction yields qualitatively. What does this mean? Generally speaking, I don't really believe in yields other than 0%, 25%, 50%, 75%, or quantitative. I also sometimes break this down as 1.)...
- A Taxol Synthesis Is Simple
I got the results back from NSF fellowship application today. I didn't get the fellowship, but that doesn't matter. Something funny was that one of the reviewers said my “proposed project looked simple or at least written...
- Column Chromatography....seems Silly Now.
Last month the team at Eisai responsible for the commercial scale synthesis of Halaven (easily the most complex drug molecule produced entirely by chemical synthesis) published a series of papers (theres three links there) detailing the development...
