Electrochemistry
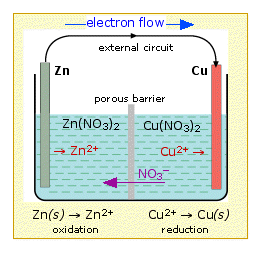
This arrangement is called a Galvanic cell. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through.
If we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when Zn2+ ions emerge from the zinc in the left cell would be able to flow through the external circuit and into the right electrode, where they could be delivered to the Cu2+ ions which become "discharged", that is, converted into Cu atoms at the surface of the copper electrode. The net reaction is the oxidation of zinc by copper(II) ions:
but this time, the oxidation and reduction steps (half reactions) take place in separate locations:
Electrochemical cells allow measurement and control of a redox reaction.
- Faraday Law
FARADAY LAWAmount of substance produce of each electrode is directly proportional to quantity of charge flowing through the cell Also called Faraday's First Law of electrolysis. CALCULATING USING FARADAY'S LAWMain stepsBalance half reaction to...
- Transport Of Charge Within The Cell
For the cell to operate, not only must there be an external electrical circuit between the two electrodes, but the two electrolytes (the solutions) must be in contact. The need for this can be understood by considering what would...
- Electrochemistry
Learning Objectives(a) describe and explain redox processes in terms of electron transfer and/or of changes in oxidation number (oxidation state) (b) define the terms: (i) standard electrode (redox) potential (ii)...
- Electrochemistry : 10.3 : Electrolysis Cell
VOLTAN CELL VS ELECTRIOLYSIS CELL . Voltaic cell :use a spontaneous reaction to generate electric energy. Electrolysis :use electric energy to drive non- spontaneous energy. VOLTAIC CELL. ELECTROLYTICelectrons generate...
- Electrochemistry : 10.1 Galvanic Cell
Electrochemistry Study of relationship between chemical change & electric work Oxidation Loss of electron by species accompanied byn an increase in oxidation number Ex: Reduction Gain electron by a species accompanied by a decrease number...
Electrochemistry
Galvanic Cells

This arrangement is called a Galvanic cell. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through.
If we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when Zn2+ ions emerge from the zinc in the left cell would be able to flow through the external circuit and into the right electrode, where they could be delivered to the Cu2+ ions which become "discharged", that is, converted into Cu atoms at the surface of the copper electrode. The net reaction is the oxidation of zinc by copper(II) ions:
Zn(s) + Cu2+ → Zn2+ + Cu(s)
but this time, the oxidation and reduction steps (half reactions) take place in separate locations:
left electrode: Zn(s) → Zn2+ + 2e– oxidation
right electrode: Cu2+ + 2e–→ Cu(s) reduction
The reaction can be started and stopped by connecting or disconnecting the two electrodes. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. By connecting a battery or other source of current to the two electrodes, we can force the reaction to proceed in its non-spontaneous, or reverse direction.
By placing an ammeter in the external circuit, we can measure the amount of electric charge that passes through the electrodes, and thus the number of moles of reactants that get transformed into products in the cell reaction.
Electric charge q is measured in coulombs. The amount of charge carried by one mole of electrons is known as the faraday, which we denote by F. Careful experiments have determined that 1 F = 96467 c. For most purposes, you can simply use 96,500 coulombs as the value of the faraday.
When we measure electric current, we are measuring the rate at which electric charge is transported through the circuit. A current of one ampere corresponds to the flow of one coulomb per second.
- Faraday Law
FARADAY LAWAmount of substance produce of each electrode is directly proportional to quantity of charge flowing through the cell Also called Faraday's First Law of electrolysis. CALCULATING USING FARADAY'S LAWMain stepsBalance half reaction to...
- Transport Of Charge Within The Cell
For the cell to operate, not only must there be an external electrical circuit between the two electrodes, but the two electrolytes (the solutions) must be in contact. The need for this can be understood by considering what would...
- Electrochemistry
Learning Objectives(a) describe and explain redox processes in terms of electron transfer and/or of changes in oxidation number (oxidation state) (b) define the terms: (i) standard electrode (redox) potential (ii)...
- Electrochemistry : 10.3 : Electrolysis Cell
VOLTAN CELL VS ELECTRIOLYSIS CELL . Voltaic cell :use a spontaneous reaction to generate electric energy. Electrolysis :use electric energy to drive non- spontaneous energy. VOLTAIC CELL. ELECTROLYTICelectrons generate...
- Electrochemistry : 10.1 Galvanic Cell
Electrochemistry Study of relationship between chemical change & electric work Oxidation Loss of electron by species accompanied byn an increase in oxidation number Ex: Reduction Gain electron by a species accompanied by a decrease number...
