Electrochemistry
reaction rate = -d[A]/dt = d[B]/dt

- 10.2 Nernst Equation
Electrochemistry : 10.2 Nernst Equation NERNST EQUATION Cell potential (E°cell) under any condition Ecell = E°cell – (RT/nF) ln Q R: universal gas constant. Q...
- Standard Hydrogen Potential
Standard Reduction PotentialsStandard reduction potential (E0) is the voltage associated with a reduction reaction at an electrode when all solutes are 1 M and all gases are at 1 atm. Standard hydrogen electrode (SHE) Reduction Reaction ...
- Galvanic Cell
Oxidation is defined as the addition of oxygen to a substancethe loss of hydrogen from a substancethe...
- Reaction Kinetic : 11.3 Factor Affecting Reaction Rate
There are 4 factors that affect the rate of reaction which are:concentrationparticle sizepressuretemperature Concentrationthe relationship between the rate of reaction and the concentration can be show below: as concentration increase,...
- Electrochemistry : 10.2 Nernst Equation
NERNST EQUATION Cell potential (E°cell) under any condition Ecell = E°cell – (RT/nF) ln Q R: universal gas constant. Q...
Electrochemistry
Reaction Kinetic : 11.1 Reaction Rate
- What is reaction rate?
- how fast the concentration of reactant(or product) are change in a chemical reaction
- How to expressing the reaction rate?
reaction rate = -d[A]/dt = d[B]/dt
- The negative value of [A] indicates the decreasing amount of A which is the reactant
- How to calculate the reaction rate ?

- How to differential the rate of reaction?aA + bB → cC + dD
- rate equation for the above reaction can be written as:
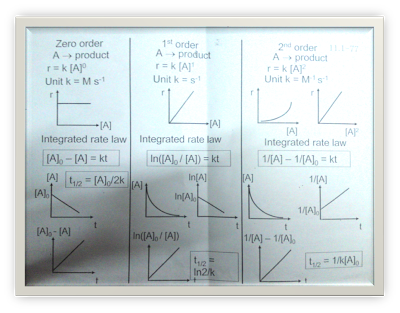
- Rate law and order of reaction

- 10.2 Nernst Equation
Electrochemistry : 10.2 Nernst Equation NERNST EQUATION Cell potential (E°cell) under any condition Ecell = E°cell – (RT/nF) ln Q R: universal gas constant. Q...
- Standard Hydrogen Potential
Standard Reduction PotentialsStandard reduction potential (E0) is the voltage associated with a reduction reaction at an electrode when all solutes are 1 M and all gases are at 1 atm. Standard hydrogen electrode (SHE) Reduction Reaction ...
- Galvanic Cell
Oxidation is defined as the addition of oxygen to a substancethe loss of hydrogen from a substancethe...
- Reaction Kinetic : 11.3 Factor Affecting Reaction Rate
There are 4 factors that affect the rate of reaction which are:concentrationparticle sizepressuretemperature Concentrationthe relationship between the rate of reaction and the concentration can be show below: as concentration increase,...
- Electrochemistry : 10.2 Nernst Equation
NERNST EQUATION Cell potential (E°cell) under any condition Ecell = E°cell – (RT/nF) ln Q R: universal gas constant. Q...
