Electrochemistry
- [shortcut] Determine No. Of Paired/ Unpaired E– In A Species E.g. Te+
← Back to Atomic Structure Determine total e– count of the species. If electron count is less than 18, it is faster to just write out the electronic configuration. If electron count is more than 18, subtract e– count by 18, 36 or 54; the largest...
- Atomic Structure
NOTES: TYPES OF QUESTIONS: Writing Electronic Configuration [Shortcut] Determine no. of paired/ unpaired e– in a species ...
- Writing Free Radical Substitution Mechanism
← Back to Alkanes When writing mechanisms: It is a good practice to write the name of the mechanism whether or not the question asks for it. You just need to show the series of equations/ arrows. There is no need for descriptions i.e. the...
- Atomic Structure
Learning Objectives (a) identify and describe protons, neutrons and electrons in terms of their relative charges and relative masses (b) deduce the behaviour of beams of protons, neutrons and electrons in both electric and magnetic fields (c) describe...
- H2 Chemistry Syllabus (2008)
PHYSICAL CHEMISTRY 1. ATOMS, MOLECULES AND STOICHIOMETRY • Relative masses of atoms and molecules • The mole, the Avogadro constant • The calculation of empirical and molecular formulae • Reacting masses and volumes (of solutions and gases) 2....
Electrochemistry
Writing Electronic Configurations
← Back to Atomic Structure
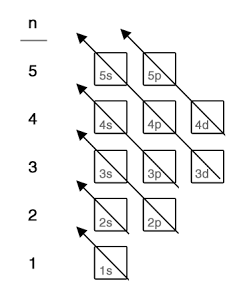
To present the electronic configuration of a species, we need to observe 3 rules/ principles in filling up the atomic orbitals:
Lowest energy first (Aufbau Principle)
- Within each subshell, fill up singly before pairing (Hund’s Rule)
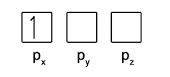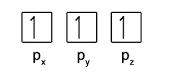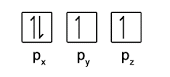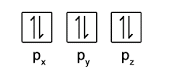
- Electrons in the same orbital must have opposite spins (Pauli Exclusion Principle)
Transition Metals [d block]
- 4s before 3d. Fill up 4s before 3d; remove from 4s before 3d.
- Note the exceptions: Cr [3d54s1] and Cu [3d104s1]
- Common mistake: When writing configuration for Transition Metal ions, students first determine the resultant no. of e– (i.e. [Fe3+] : 23 e–) before filling up the orbitals i.e. [Incorrect]1s2 2s2 2p6 3s2 3p6 3d3 4s2. Instead, we should write the configuration for the element first and then remove the required no. of e– from the 3d orbitals. i.e. [Correct]1s2 2s2 2p6 3s2 3p6 3d5.
[FAQ] When writing electron configuration, do I just write 2p4 or do I need to show 2px2 2py1 2pz1?
When asked for electron configuration, you do not have to write out the subshells e.g. O : 1s2 2s2 2p4
BUT to explain for certain trends related to how the e– are placed in the subshells (e.g. ionisation energy), you NEED to show how the e– are distributed in px, py and pz e.g. O: 1s2 2s2 2px 2 2py1 2pz1
- [shortcut] Determine No. Of Paired/ Unpaired E– In A Species E.g. Te+
← Back to Atomic Structure Determine total e– count of the species. If electron count is less than 18, it is faster to just write out the electronic configuration. If electron count is more than 18, subtract e– count by 18, 36 or 54; the largest...
- Atomic Structure
NOTES: TYPES OF QUESTIONS: Writing Electronic Configuration [Shortcut] Determine no. of paired/ unpaired e– in a species ...
- Writing Free Radical Substitution Mechanism
← Back to Alkanes When writing mechanisms: It is a good practice to write the name of the mechanism whether or not the question asks for it. You just need to show the series of equations/ arrows. There is no need for descriptions i.e. the...
- Atomic Structure
Learning Objectives (a) identify and describe protons, neutrons and electrons in terms of their relative charges and relative masses (b) deduce the behaviour of beams of protons, neutrons and electrons in both electric and magnetic fields (c) describe...
- H2 Chemistry Syllabus (2008)
PHYSICAL CHEMISTRY 1. ATOMS, MOLECULES AND STOICHIOMETRY • Relative masses of atoms and molecules • The mole, the Avogadro constant • The calculation of empirical and molecular formulae • Reacting masses and volumes (of solutions and gases) 2....
