Electrochemistry
- Making C–c Bonds With The Simplicity Of Making Amide Bonds
Despite all of the wonderful advances in organic synthesis, amide-bond formation is still the most widely used reaction among those making medicines. This truth has led some to declare their embarrassment and depression. Medicinal...
- Hey! We Used C-h Activation To Make A Natural Product!
Once upon a time, we were interested making fumitremorgin A... Phil's interest in verruculogen and fumitremorgin A has been long standing (see: Austamine and Okaramine), but strangely the endoperoxide-containing indole alkaloids haven't...
- Formal Olefin Hydroamination With Nitroarenes
Our latest work in the field of iron chemistry came out today in Science. First we would like to present a graph (shown below) on how this newly developed chemistry could simplify the synthesis of some biologically active intermediates: starting from...
- Functionalized Olefin Cross-coupling
Figure 1. Functionalized olefin cross-coupling at a glance. The last paper of the year from the inner depths of our lab has made its way out earlier today. It’s the bigger brother of our reductive olefin coupling paper from January. In a nutshell, reductive...
- Diterpenoid-alkaloids Are So...fancy (you Already Know)
The second paper on our efforts toward diterpenes and related diterpenoid-alkaloids is out in JACS now. This is building off of work previously published in Angewante last year. The story of this project goes back more than 5 years. ...
Electrochemistry
A Tale of Two Olefins
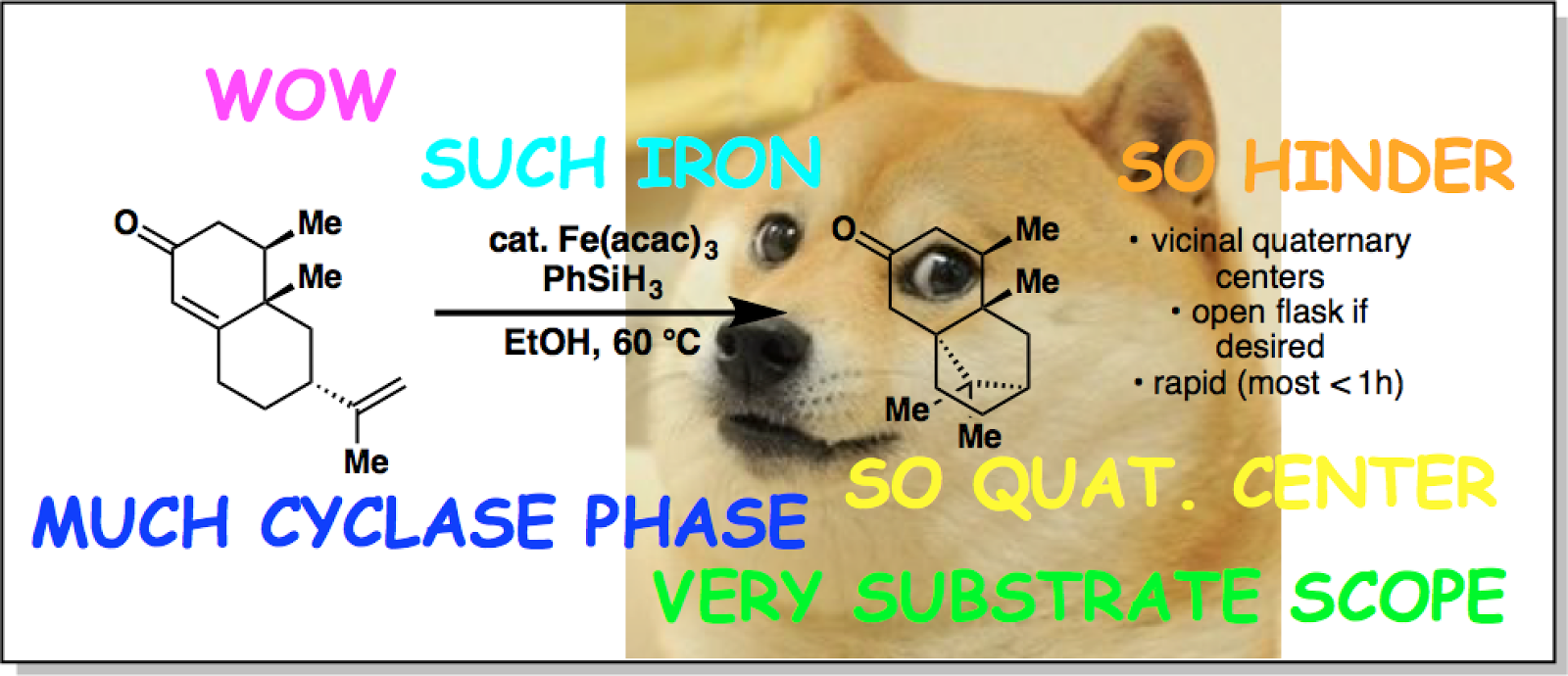 |
| A prototype of the graphical abstract that Phil didn't want to submit to JACS. |
Today marks the day that another paper makes its way out of the hot little presses of the ACS Publication Office and into the world of chemistry. This time, it's a paper from our lab that details a reductive coupling of an election-neutral olefin with an electron-deficient one. Since the chemistry is already described in the article, I wanted give an account of how this project came to be.
Apparently Phil's gotten at lot of flak regarding the two-phase approach to terpene total synthesis. To be frank, the oxidase phases of our projects get significantly more attention by the general synthetic community than the accompanying cyclase phases. Some of the people in our lab even choose to replace the cyclase phase with a "purchase" phase so they can get right into the meat of the oxidase phase. In my first months here, I was talking to Emily (my hoodmate) and she said something to the effect of "no matter how you look at it, C–H oxidations are sexy."
Meanwhile, some would argue that the cyclase phases of our projects are simply comprised of Robinson annulations, conjugate additions, Diels-Alder cycloadditions, polyene cyclizations, ect.—"tried and true" reactions that your eye quickly passes over as you look for the unusual transformations in the synthesis. It would be pretty cool if we could breathe some extra pizazz into our cyclase phases and make them just as exciting as our oxidase phases.
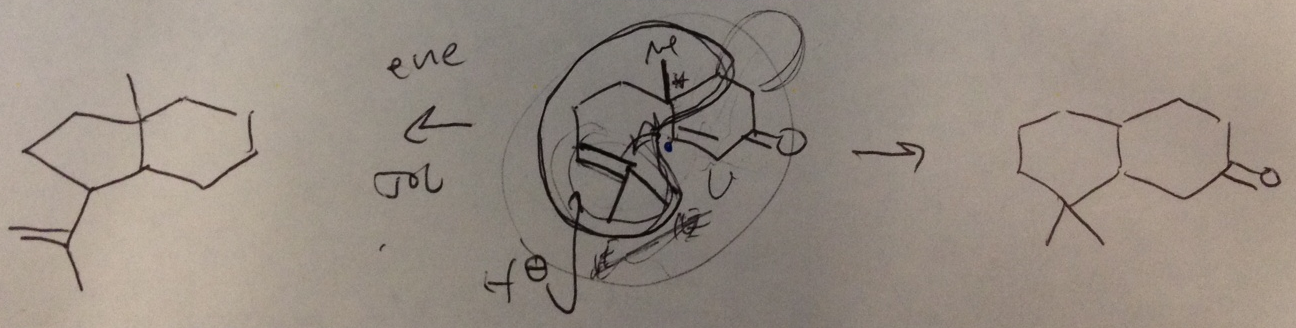 |
| Phil's musings on olefin cyclizations. |
Phil outlined this all for me one day when I popped in his office to talk about new projects. At some point he sketched some structures that I've included above to help illustrate an idea he had to sexify our cyclase phases (I know there's a carbonyl and a methyl missing from the left- and rightmost structures, respectively, but I think we can forgive him). To the left is a cyclization of an enone that Snider was able to coax using EtAlCl₂ as a Lewis acid to give a bicyclo[4.3.0]nonane. The decalin to the right is a pretty recognizable motif in terpenes that Phil wanted to synthesize from the readily available starting material that Snider used. Like Snider's transformation, Phil wanted a single-step reaction where we wouldn't have to convert the olefins to other functional groups in order to achieve the cyclization.
I set out trying to achieve this transformation using conditions that were inspired by a paper from Boger's group and was later joined by Yuki-san (rock-star postdoc and basketball extraordinaire). We were able to extend the method to intermolecular couplings and the rest of the story is history, or rather, outlined in our paper. The end result is a reaction that's pretty easy to set up—it's literally a dump and stir reaction. I've even made a short video of it below for those who want a little extra convincing.
There's one last thing I wanted to mention involving the mechanism we proposed at the end of the paper. Although we outlined some mechanistic probes in the text of the paper, there were a couple of others that we did not include that appear to support the formation of radical intermediates. When the vinyl cyclopropane shown below was subjected to the reaction conditions, I was able to observe several products by GC/MS: starting material and another product with m/z = 144, four separate products with m/z = 146, and one product with m/z = 148. Some possible structures that fit these criteria are shown below. However, I was not able to isolate and individually characterize these products.
 |
| Vinylcyclopropane experiment and potential structures of the products. |
It also would have been nice to obtain an EPR spectrum of a mixture of Fe(acac)₃ and PhSiH₃ to see if we could observe an Fe(III) hydride, but Scripps unfortunately does not possess an EPR spectrometer.
Anyways, if anyone has questions about the project or any of the background behind it, I'd be more than happy to answer them!
- Making C–c Bonds With The Simplicity Of Making Amide Bonds
Despite all of the wonderful advances in organic synthesis, amide-bond formation is still the most widely used reaction among those making medicines. This truth has led some to declare their embarrassment and depression. Medicinal...
- Hey! We Used C-h Activation To Make A Natural Product!
Once upon a time, we were interested making fumitremorgin A... Phil's interest in verruculogen and fumitremorgin A has been long standing (see: Austamine and Okaramine), but strangely the endoperoxide-containing indole alkaloids haven't...
- Formal Olefin Hydroamination With Nitroarenes
Our latest work in the field of iron chemistry came out today in Science. First we would like to present a graph (shown below) on how this newly developed chemistry could simplify the synthesis of some biologically active intermediates: starting from...
- Functionalized Olefin Cross-coupling
Figure 1. Functionalized olefin cross-coupling at a glance. The last paper of the year from the inner depths of our lab has made its way out earlier today. It’s the bigger brother of our reductive olefin coupling paper from January. In a nutshell, reductive...
- Diterpenoid-alkaloids Are So...fancy (you Already Know)
The second paper on our efforts toward diterpenes and related diterpenoid-alkaloids is out in JACS now. This is building off of work previously published in Angewante last year. The story of this project goes back more than 5 years. ...
