Electrochemistry
Phil's interest in verruculogen and fumitremorgin A has been long standing (see: Austamine and Okaramine), but strangely the endoperoxide-containing indole alkaloids haven't seen any attention despite the long history of syntheses of related natural products, including the (spiro)trypostatins, brevianamides, and other tryptophan-proline-diketopiperaizne alkaloids. Around '03 or '04, while I was toiling away in high school, a graduate student, Ben Hafensteiner, did a few reactions on the project before falling deep into the hole of a related family, the stephacidins. So the fumitremorgin project lay dormant for quite some time, before I took it over in 2011 (or was it '12? I don't even remember).
So in this post, I mainly want to talk about weird stuff, or stuff that didn't work; things not in the paper. Some are elaborations that were included in my thesis. I'm also much more likely to talk about things I already have schemes for. Its my blog post. I do want I want.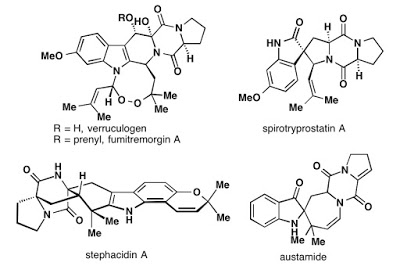
- Making C–c Bonds With The Simplicity Of Making Amide Bonds
Despite all of the wonderful advances in organic synthesis, amide-bond formation is still the most widely used reaction among those making medicines. This truth has led some to declare their embarrassment and depression. Medicinal...
- Formal Olefin Hydroamination With Nitroarenes
Our latest work in the field of iron chemistry came out today in Science. First we would like to present a graph (shown below) on how this newly developed chemistry could simplify the synthesis of some biologically active intermediates: starting from...
- Academia–industry Collaboration In The Route Optimization Of Taxadienone
In early 2012, we reported the gram-scale synthesis of a non-natural taxane, taxadienone, as well as that of a natural taxane, taxadiene. Now, chemists at Albany Molecular Research Inc. (AMRI) report a route optimization of taxadienone in none other than...
- Ingenol: Behind The Scenes
Disappointment, frustration, excitement, setbacks, thrill, and success. It has been a roller-coaster ride, but finally the reward is here. A total synthesis of ingenol from our lab was published online today. The chemistry is all described in the paper,...
- Group Member Of This Blog
Group 1 ~Thermochemistry~ Group Leader : Khor Sheng Yau Muhammad Amirul Syafiq Bin Azudin ...
Electrochemistry
Hey! We used C-H activation to make a natural product!
Once upon a time, we were interested making fumitremorgin A...
Phil's interest in verruculogen and fumitremorgin A has been long standing (see: Austamine and Okaramine), but strangely the endoperoxide-containing indole alkaloids haven't seen any attention despite the long history of syntheses of related natural products, including the (spiro)trypostatins, brevianamides, and other tryptophan-proline-diketopiperaizne alkaloids. Around '03 or '04, while I was toiling away in high school, a graduate student, Ben Hafensteiner, did a few reactions on the project before falling deep into the hole of a related family, the stephacidins. So the fumitremorgin project lay dormant for quite some time, before I took it over in 2011 (or was it '12? I don't even remember).
So in this post, I mainly want to talk about weird stuff, or stuff that didn't work; things not in the paper. Some are elaborations that were included in my thesis. I'm also much more likely to talk about things I already have schemes for. Its my blog post. I do want I want.
As I wound down my work on a previous project, I was looking around for something to work on for my last years of graduate school... after a few forays into bad ideas, including a comically convoluted, terrible, imidazolidine synthesis and a proposal to work on cubane, Phil suggested taking a look at fumitremorgin A. From the start, this molecule wasn't love at first sight. It was nothing like any of the molecules I was attracted to: typically compact, caged, multi-stereocentered, terpenes (e.g. vinigrol or maoecrystal). I chose to work on it anyway.
Although it was not quite to my tastes, it was intriguing: the peroxide was neat. There's a medium sized ring; and the peroxyhemiaminal-thing is funky. Hmm... late stage oxidation probably won't work in the presence of the indole. Oh... you can't buy 6-methoxytryptophan? That complicates things too. So I agreed to work on it. I estimated we'd be done in about a year. No big deal, this class of molecules had seen a lot of work.
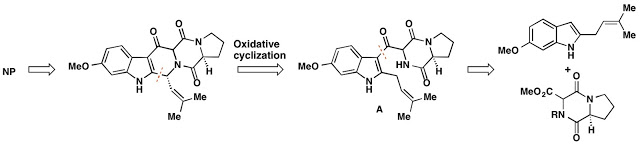
I started my journey with Will Gutekunst, a fourth year graduate student who worked on a multitude of successful projects. We worked on a disconnection for the molecule that looked approximately like this:
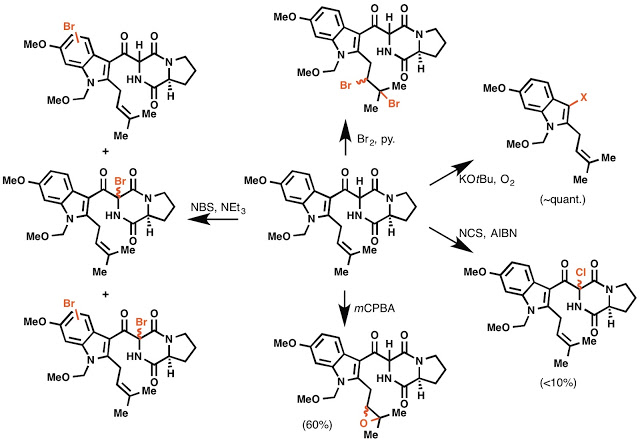
Unfortunately, that retrosynthetic disconnection failed completely. We were never able to realize this transformation from compound A to the pentacycle without over-oxidation of the molecule. What that looks like in practice is a whole lot of reactions that were not characterizable by NMR (so called "nonspecific decomposition"), as well as the following scheme:
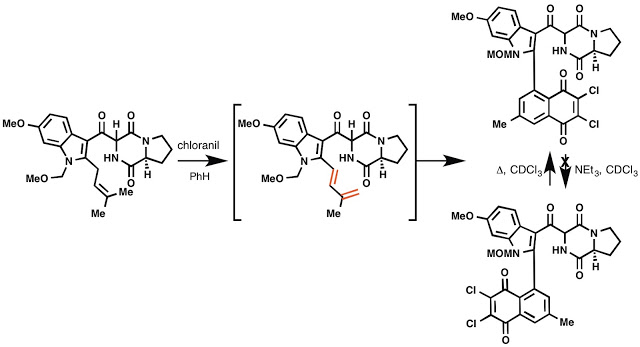
I thought this next oxidation was kind of neat. I once made these two isolable rotomers, which were separable by PTLC and could be characterized fully by NMR. The fact that they convert during heating, but not with base, was suggestive of rotomers. I never really fully explored this, since I made only a few mg of material. Oh well, I still think its cool.
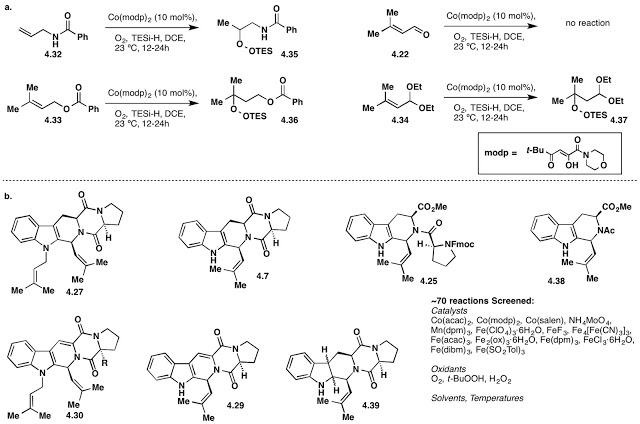
Eventually, Will moved on to brighter pastures, and I turned my attention to one of the bigger problems, the endoperoxide. From the get-go, we had though late stage oxidation would be an issue -- and the work Will and I conducted made this seem likely. We assumed that the peroxide could be installed early from O2 and a Mukaiyama oxidation, or perhaps through the addition of peroxide into an aldehyde. Ultimately, we found that the Mukaiyama peroxidation of prenyl aldehyde diethyl acetal was a high yielding, and reproducible method for forming a "prenyl peroxide." We did explore other conditions for this transformation involving a variety of metals (notably Co, Mn, and Fe), as well as the use of H2O2 with (and without) acid. Ultimately, the Mukaiyama reaction, one of the first we looked at for peroxide installation, worked well. During this time, the blast shield was kept on-hand, along with peroxide test strips and a lot of reducing agent. There were never any issues working with peroxides in our hands, but as always, take care, don't concentrate to dryness, and know what you're doing, please.
Here's a scheme I'm self plagiarizing from my thesis (deal with it). The first reaction was one of Mukayiama's own. Then we slowly worked our way to the final reaction (to make 4.37). In the paper, you'll notice we had to swap the TES- for a TBDPS-group. Its worth mentioning that you can put other silanes into this peroxidation (like TBSi-H) and achieve the same reaction. However, bulkier silanes (TIPSi-H and TBDPSi-H) failed to produce peroxide in our hands... hence the protecting group swap. The TBDPS-peroxide functioned well in the Pictet-Spengler reaction as well as the PhNO/ZrCl4 mediated unsaturation, so we needed it. Protecting groups, yay!
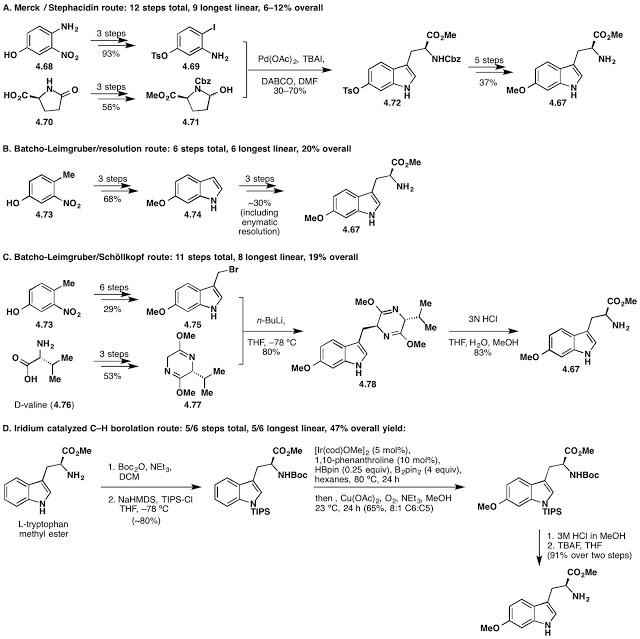
At some point in the aforementioned ramblings, was joined on the project by Feng Yu and Jochen Zoller, a postdoc and visiting graduate student, respectively. Feng was tasked with something I had barely touched: a better 6-methoxytrpytophan synthesis. Until this point in time, I had only been using known methods to form my methoxytryptophan, and while each of these worked, they were time consuming syntheses in their own right (see scheme below for synthetic detail). So Feng really dug into the literature to come up with something better. After a while, he came up with a proposal for a C-H oxidation methodology that, in the end, looked a lot like what was published in our paper. I tip my hat to Feng for the dedication to stick with this method, because I was busy playing with the peroxide while Feng was taking multiple steps off the synthesis and developing the method fully! I've got a decent comparison of Feng's method to to other known literature methods (below), but you should check the paper for the full method details. He'll likely chime in in the comments if anyone has any questions. BTW, 47% yield to methoxytryptophan more than doubles the literature standard (~20%)!
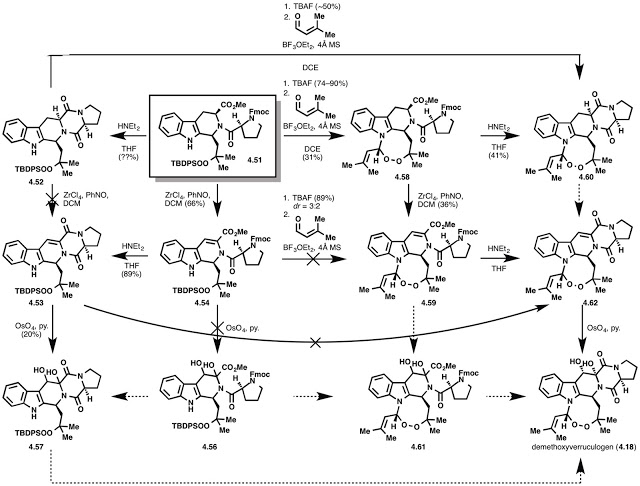
While Feng was busy with methoxytryptophan, Jochen and I were working out the final sequence of our synthesis of demethoxyverruculogen (4.18 below). I'll let the following scheme speak for itself, but suffice it to say, we tried almost ever possible iteration to achieve a sequence that worked.
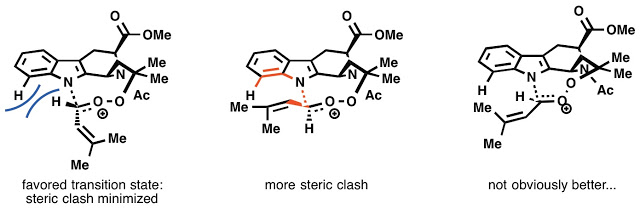
The reason for a single (or close to it) diastereomer of the 8-membered endoperoxide forming remains unknown. I played with a lot of models and drew a lot of schemes to try and rationalize it. Here are some ideas. Maybe you have a better one?
With that final result (demethoxyverruculogen) in hand, Feng, Jochen, and I pushed the last remaining bit of material (methoxytryptophan) forward to complete the synthesis of verruculogen. The NMR was completely terrible (think: 600 MHz cryoprobe, proton NMR, over the weekend, impure), even after purification by PTLC the size of a TLC plate (2 cm x 4 cm). Actually, the only way we were able to even find the natural product on TLC, NMR, or LC/MS is because I previously had grown the fungus in our lab for natural product isolation and stability studies. That's right, the Baran Lab does "biology." Sort of. Many thanks to Van Vu of the Marletta Lab and Erik Wold of the Felding Lab for helping me successfully total biologize.
This was last summer! Then I graduated and peaced out of San Diego.
Since that time, Leah Simke and Shigenobu Umemiya joined the project and Feng Yu has remained working on it. They optimized the entire sequence and tweaked the final steps to make them work better (switch reagents and order). In addition, fumitremorgin A was synthesized (verruculogen+prenyl group, see the first scheme).
What I mean to say is, a lot of people deserve credit for work done here. I mean to thank them. Finally, I would like to thank coffee, without you I would have probably slept more.
- Making C–c Bonds With The Simplicity Of Making Amide Bonds
Despite all of the wonderful advances in organic synthesis, amide-bond formation is still the most widely used reaction among those making medicines. This truth has led some to declare their embarrassment and depression. Medicinal...
- Formal Olefin Hydroamination With Nitroarenes
Our latest work in the field of iron chemistry came out today in Science. First we would like to present a graph (shown below) on how this newly developed chemistry could simplify the synthesis of some biologically active intermediates: starting from...
- Academia–industry Collaboration In The Route Optimization Of Taxadienone
In early 2012, we reported the gram-scale synthesis of a non-natural taxane, taxadienone, as well as that of a natural taxane, taxadiene. Now, chemists at Albany Molecular Research Inc. (AMRI) report a route optimization of taxadienone in none other than...
- Ingenol: Behind The Scenes
Disappointment, frustration, excitement, setbacks, thrill, and success. It has been a roller-coaster ride, but finally the reward is here. A total synthesis of ingenol from our lab was published online today. The chemistry is all described in the paper,...
- Group Member Of This Blog
Group 1 ~Thermochemistry~ Group Leader : Khor Sheng Yau Muhammad Amirul Syafiq Bin Azudin ...
