Electrochemistry






Cheers
- Making C–c Bonds With The Simplicity Of Making Amide Bonds
Despite all of the wonderful advances in organic synthesis, amide-bond formation is still the most widely used reaction among those making medicines. This truth has led some to declare their embarrassment and depression. Medicinal...
- Hey! We Used C-h Activation To Make A Natural Product!
Once upon a time, we were interested making fumitremorgin A... Phil's interest in verruculogen and fumitremorgin A has been long standing (see: Austamine and Okaramine), but strangely the endoperoxide-containing indole alkaloids haven't...
- Formal Olefin Hydroamination With Nitroarenes
Our latest work in the field of iron chemistry came out today in Science. First we would like to present a graph (shown below) on how this newly developed chemistry could simplify the synthesis of some biologically active intermediates: starting from...
- Diterpenoid-alkaloids Are So...fancy (you Already Know)
The second paper on our efforts toward diterpenes and related diterpenoid-alkaloids is out in JACS now. This is building off of work previously published in Angewante last year. The story of this project goes back more than 5 years. ...
- A Tale Of Two Olefins
A prototype of the graphical abstract that Phil didn't want to submit to JACS.Today marks the day that another paper makes its way out of the hot little presses of the ACS Publication Office and into the world of chemistry. This time, it's a...
Electrochemistry
Ingenol: Behind the scenes

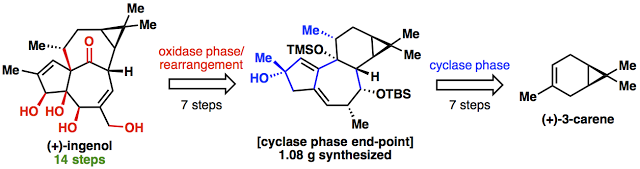
Disappointment, frustration, excitement, setbacks, thrill, and success. It has been a roller-coaster ride, but finally the reward is here. A total synthesis of ingenol from our lab was published online today. The chemistry is all described in the paper, so I will not go into too much detail here. However, I want to highlight some of the key reactions and hurdles we had to overcome to finally being able to publish this work.
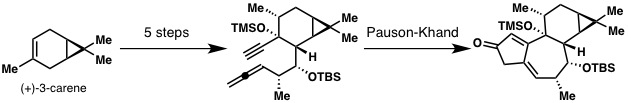
Our synthesis starts from commercially available and cheap (+)-3-carene, and in five steps we get to the substrate for the first key reaction, the Pauson-Khand. In order to get enough of the Pauson-Khand product, we needed to optimize this reaction quite a bit, but we can run it on gram-scale now as shown below.



Gram-scale Pauson-Khand reaction
1,2-Addition of MeMgBr to the carbonyl group of the P-K product gives us the so-called cyclase phase end-point.

Happy Christian with 1 gram of cyclase-phase endpoint
The oxidase phase also consists of seven steps, in which we install the four hydroxyl groups and rearrange the tigliane skeleton to give the desired ingenane skeleton.
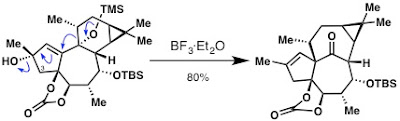
The most troublesome, frustrating, and time-consuming problem to overcome was the pinacol shift to give the ingenane skeleton.

After 8 months of desperation, numerous shift reactions in one form or the other, a lot of disappointing results, and a ridiculous amount of NMR time to figure out what we got from all these reactions, we still couldn’t get the desired product. In fact, we actually almost abandoned the whole route because we just couldn’t get that shift to work the way we wanted. Eventually, we agreed to start designing another route to ingenol, if we could not get the shift to work by November 1st, 2012.
You may call it insane luck, but on October 24th 2012 we finally hit the sweet spot and got the reaction to work on a model substrate and about a week later on the real system. What a relief! Finally we could move on.
Comparably, the last steps of the synthesis were a much smoother ride. An allylic oxidation, a deprotection, and an elimination step later we eventually had a tiny amount of our first natural product, 20-deoxyingenol, just before Christmas of 2012. About a month later, we finally made ingenol for the first time.
However, it took us another 6 months of optimization and scale-up to get the end result, which was published online on Science Express today.
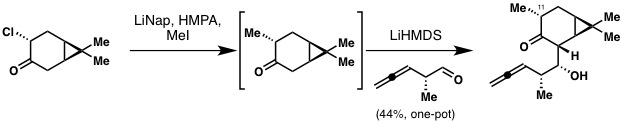
Especially, the reductive alkylation of the chloro-ketone needed some serious optimization. Initially, we tried to prepare big quantities of the methyl ketone itself which turned out to be very difficult to prepare and handle. We had some really painful weeks where we would run this reaction almost everyday and get out nearly nothing. At some point, Phil suggested to run both steps - reductive methylation and aldol reaction - in one pot (!) without isolating the methyl ketone itself. Initially we had a hard time imagining that this would work but after some optimization we finally arrived at the one-pot methylation aldol procedure.


Frustration after another failed attempt at the reductive methylation


What do you do if you almost go nuts? You do stuff like drawing your team-mates.
Here is Christian’s artistic interpretation of Steve and myself.
So what’s up next? We are currently collecting data for an upcoming full paper, which will cover the side reactions and failed approaches. Furthermore, we are focusing on making analogs of ingenol for pharmacological testing. The key intermediate, the cyclase phase end-point, which is our branch point for analog syntheses is being scaled–up in conjunction with our collaborators at LEO Pharma, developers of Picato® (an ester of ingenol).

Whoever is reading this post, if any, please feel free to comment and ask us questions about the synthesis. We will be more than happy to answer them and give you more insights.
Cheers
- Making C–c Bonds With The Simplicity Of Making Amide Bonds
Despite all of the wonderful advances in organic synthesis, amide-bond formation is still the most widely used reaction among those making medicines. This truth has led some to declare their embarrassment and depression. Medicinal...
- Hey! We Used C-h Activation To Make A Natural Product!
Once upon a time, we were interested making fumitremorgin A... Phil's interest in verruculogen and fumitremorgin A has been long standing (see: Austamine and Okaramine), but strangely the endoperoxide-containing indole alkaloids haven't...
- Formal Olefin Hydroamination With Nitroarenes
Our latest work in the field of iron chemistry came out today in Science. First we would like to present a graph (shown below) on how this newly developed chemistry could simplify the synthesis of some biologically active intermediates: starting from...
- Diterpenoid-alkaloids Are So...fancy (you Already Know)
The second paper on our efforts toward diterpenes and related diterpenoid-alkaloids is out in JACS now. This is building off of work previously published in Angewante last year. The story of this project goes back more than 5 years. ...
- A Tale Of Two Olefins
A prototype of the graphical abstract that Phil didn't want to submit to JACS.Today marks the day that another paper makes its way out of the hot little presses of the ACS Publication Office and into the world of chemistry. This time, it's a...
