Electrochemistry
An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. This reaction may take place in a single electron-transfer step, or as a succession of two or more steps. The substances that receive and lose electrons are called the electroactive species.
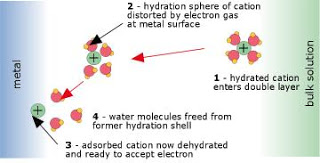
Fig. 4: Electron transfer at an anode
and the half-cell reaction would be
The reaction occurs at the surface of the electrode (Fig 4 above). The electroactive ion diffuses to the electrode surface and adsorbs (attaches) to it by van der Waals and coulombic forces. In doing so, the waters of hydration that are normally attached to any ionic species must be displaced. This process is always endothermic, sometimes to such an extent that only a small fraction of the ions be able to contact the surface closely enough to undergo electron transfer, and the reaction will be slow. The actual electron-transfer occurs by quantum-mechanical tunnelling.
The half cell would be represented as
- Standard Half-cell Potentials
Problem Example 1 Find the standard potential of the cell Cu(s) | Cu2+ || Cl– | AgCl(s) | Ag(s)and predict the direction of electron flow when the two electrodes are connected. Solution: The net reaction corresponding to this cell will be 2 Ag(s) +...
- Galvanic Cells
This arrangement is called a Galvanic cell. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The two...
- Electrochemistry : 10.3 : Electrolysis Cell
VOLTAN CELL VS ELECTRIOLYSIS CELL . Voltaic cell :use a spontaneous reaction to generate electric energy. Electrolysis :use electric energy to drive non- spontaneous energy. VOLTAIC CELL. ELECTROLYTICelectrons generate...
- Electrochemistry : 10.1 Galvanic Cell (continued)
SPONTANEOUS REACTION occurs as the result of different ability of metal to give up their electron to flow through the circuit. CELL POTENTIAL (ECell) different in electrical potential of electrodes also called voltage or electromotive...
- Electrochemistry : 10.1 Galvanic Cell
Electrochemistry Study of relationship between chemical change & electric work Oxidation Loss of electron by species accompanied byn an increase in oxidation number Ex: Reduction Gain electron by a species accompanied by a decrease number...
Electrochemistry
Electrodes and electrode reactions.
An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. This reaction may take place in a single electron-transfer step, or as a succession of two or more steps. The substances that receive and lose electrons are called the electroactive species.

This process takes place within the very thin interfacial region at the electrode surface, and involves quantum-mechanical tunneling of electrons between the electrode and the electroactive species. The work required to displace the H2O molecules in the hydration spheres of the ions constitutes part of the activation energy of the process.
In the example of the Zn/Cu cell we have been using, the electrode reaction involves a metal and its hydrated cation; we call such electrodes metal-metal ion electrodes. There are a number of other kinds of electrodes which are widely encountered in electrochemistry and analytical chemistry.Ion-ion electrodes
Many electrode reactions involve only ionic species, such as Fe2+ and Fe3+. If neither of the electroactive species is a metal, some other metal must serve as a conduit for the supply or removal of electrons from the system. In order to avoid complications that would arise from electrode reactions involving this metal, a relatively inert substance such as platinum is commonly used. Such a half cell would be represented asPt(s) | Fe3+(aq), Fe2+(aq) || ...
Fe2+(aq) → Fe3+ (aq) + e–
Gas electrodes
Some electrode reactions involve a gaseous species such as H2, O2, or Cl2. Such reactions must also be carried out on the surface of an electrochemically inert conductor such as platinum. A typical reaction of considerable commercial importance isCl–(aq) → ½ Cl2(g) + e–
Similar reactions involving the oxidation of Br2 or I2 also take place at platinum surfaces.
Insoluble–salt electrodes
A typical electrode of this kind consists of a silver wire covered with a thin coating of silver chloride, which is insoluble in water. The electrode reaction consists in the oxidation and reduction of the silver:AgCl(s) + e– → Ag(s) + Cl–(aq)
... || Cl– (aq) | AgCl (s) | Ag (s)
- Standard Half-cell Potentials
Problem Example 1 Find the standard potential of the cell Cu(s) | Cu2+ || Cl– | AgCl(s) | Ag(s)and predict the direction of electron flow when the two electrodes are connected. Solution: The net reaction corresponding to this cell will be 2 Ag(s) +...
- Galvanic Cells
This arrangement is called a Galvanic cell. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The two...
- Electrochemistry : 10.3 : Electrolysis Cell
VOLTAN CELL VS ELECTRIOLYSIS CELL . Voltaic cell :use a spontaneous reaction to generate electric energy. Electrolysis :use electric energy to drive non- spontaneous energy. VOLTAIC CELL. ELECTROLYTICelectrons generate...
- Electrochemistry : 10.1 Galvanic Cell (continued)
SPONTANEOUS REACTION occurs as the result of different ability of metal to give up their electron to flow through the circuit. CELL POTENTIAL (ECell) different in electrical potential of electrodes also called voltage or electromotive...
- Electrochemistry : 10.1 Galvanic Cell
Electrochemistry Study of relationship between chemical change & electric work Oxidation Loss of electron by species accompanied byn an increase in oxidation number Ex: Reduction Gain electron by a species accompanied by a decrease number...
