Electrochemistry
- Determine Products Of Electrophilic Addition Reactions
← Back to Alkenes The electrophile (species with + or δ+) is added to the C=C first The electrophile is added to the C with more H (Markonikov’s Rule) Presence of competing nucleophiles can lead to a mixture of products...
- Determine Type Of Bonding For Simple Molecules
← Back to Chemical Bonding To determine the type of molecule (polar/ non-polar) and IMF, you will need these four steps: 1) Draw dot-cross diagram: 1 Determine the central atom and surrounding atoms. Central...
- Oxidation Number
← Back to Electrochemistry Oxidation numbers are arbitrary numbers assigned to atoms to describe their relative state of oxidation or reduction i.e. how oxidised or reduced they are. They are assigned according to an arbitrary set of rules:...
- Introduction To Organic Chemistry : 12.5 Organic Reaction
TYPE OF CLEAVAGE : 2 type of bond cleavage:- Hemolytic cleavageHeterolytic cleavage Hemolytic cleavage The breaking of a single (two-electron) bond in which one electron remains on each of the atoms. Also known as free-radical reaction; homolysis. ...
- Thermochemistry : 9.3 Born-haber Cycle
First Ionization Energy (IE1)Energy required for 1 mol of gaseous atom to lose 1 mol of electrons. Affinity Electron (EA) Energy change that occurs when 1 mol of gaseous atom gains 1 mol of electrons. Lattice EnergyEnergy change when 1 mol of solid...
Electrochemistry
How to Draw Dot-Cross Diagram
← Back to Chemical Bonding
| 1 | Determine the central atom and surrounding atoms. Central atom is commonly the: - Element with the lesser no. of atoms - First element in chemical formula (except H) - Least electronegative element | ||||||
| 2 | For polyatomic ions: Anion: add the e– to the most electro-ve atom (one e– per atom) Cation: remove the e– from the least electro-ve element Ignore this step for neutral molecules | ||||||
| 3 | Determine no. of bonds each surrounding atom can form:
| ||||||
| 4 | Draw all the bonds to the central atom. For atoms with 8 e– (or 2 for H): form dative bond from surrounding atom to central atom For atoms with 0 e–: form dative bond from central atom to surrounding atom | ||||||
| 5 | Assign remaining e– on central atom as lone pairs. If central atom is in Period 2 and no. of e– around the atom is > 8, convert a double bond to a dative bond from the central atom to surrounding atom. |
Examples:
SO2
| 1 | Central atom: S Surrounding atoms: O |
| 2 | N.A. |
| 3 | 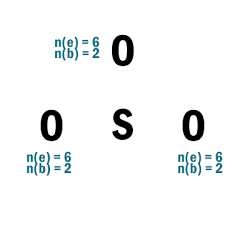 |
| 4 | 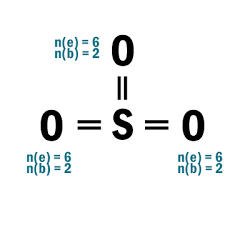 |
| 5 | N.A. (S is in P3; can expand beyond octet) |
HCN
| 1 | Central atom: C Surrounding atoms: H and N |
| 2 | N.A. |
| 3 | 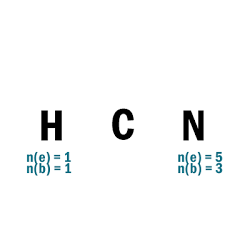 |
| 4 | 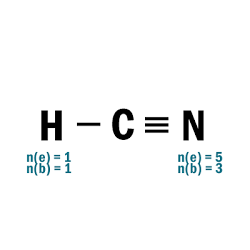 |
| 5 | N.A. |
NH3
| 1 | Central atom: N Surrounding atoms: H |
| 2 | N.A. |
| 3 | 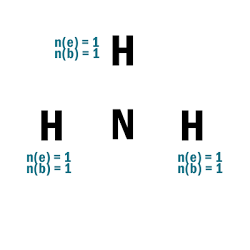 |
| 4 | 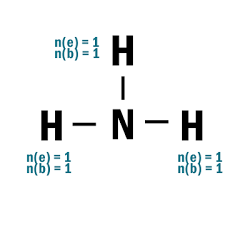 |
| 5 | N has 5 e– and 3 bonds > it has 2 remaining e–: 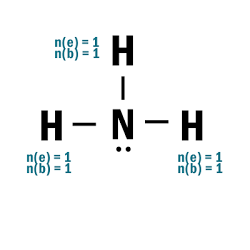 |
NO3–
| 1 | Central atom: N Surrounding atoms: O |
| 2 | Add one e– to one O atom. |
| 3 | 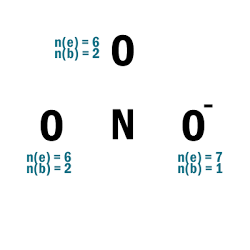 |
| 4 | 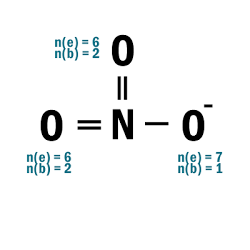 |
| 5 | N has 10e– > convert one double bond to dative bond from N to O: 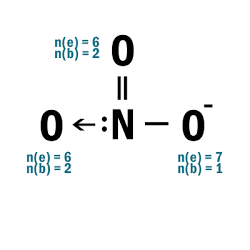 |
AlH4–
| 1 | Central atom: Al Surrounding atoms: H |
| 2 | Add one e– to one H atom. |
| 3 | 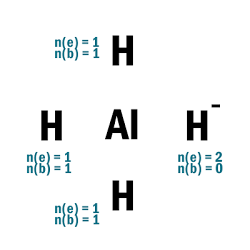 |
| 4 | 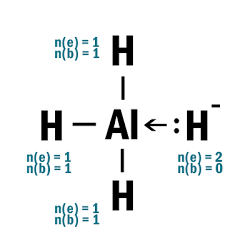 |
| 5 | N.A. |
NH4+
| 1 | Central atom: N Surrounding atoms: H |
| 2 | Remove one e– from one H atom. |
| 3 | 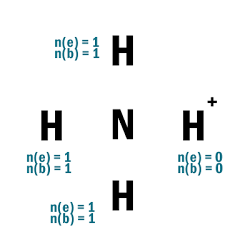 |
| 4 | 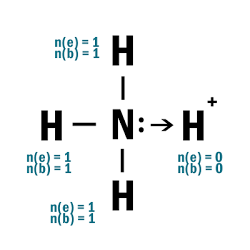 |
| 5 | N.A. |
- Determine Products Of Electrophilic Addition Reactions
← Back to Alkenes The electrophile (species with + or δ+) is added to the C=C first The electrophile is added to the C with more H (Markonikov’s Rule) Presence of competing nucleophiles can lead to a mixture of products...
- Determine Type Of Bonding For Simple Molecules
← Back to Chemical Bonding To determine the type of molecule (polar/ non-polar) and IMF, you will need these four steps: 1) Draw dot-cross diagram: 1 Determine the central atom and surrounding atoms. Central...
- Oxidation Number
← Back to Electrochemistry Oxidation numbers are arbitrary numbers assigned to atoms to describe their relative state of oxidation or reduction i.e. how oxidised or reduced they are. They are assigned according to an arbitrary set of rules:...
- Introduction To Organic Chemistry : 12.5 Organic Reaction
TYPE OF CLEAVAGE : 2 type of bond cleavage:- Hemolytic cleavageHeterolytic cleavage Hemolytic cleavage The breaking of a single (two-electron) bond in which one electron remains on each of the atoms. Also known as free-radical reaction; homolysis. ...
- Thermochemistry : 9.3 Born-haber Cycle
First Ionization Energy (IE1)Energy required for 1 mol of gaseous atom to lose 1 mol of electrons. Affinity Electron (EA) Energy change that occurs when 1 mol of gaseous atom gains 1 mol of electrons. Lattice EnergyEnergy change when 1 mol of solid...
