Electrochemistry
In most electrochemical experiments our interest is concentrated on only one of the electrode reactions. Since all measurements must be on a complete cell involving two electrode systems, it is common practice to employ a reference electrode as the other half of the cell. The major requirements of a reference electrode are that it be easy to prepare and maintain, and that its potential be stable. The last requirement essentially means that the concentration of any ionic species involved in the electrode reaction must be held at a fixed value. The most common way of accomplishing this is to use an electrode reaction involving a saturated solution of an insoluble salt of the ion. One such system, the silver-silver chloride electrode has already been mentioned:
This electrode usually takes the form of a piece of silver wire coated with AgCl. The coating is done by making the silver the anode in an electrolytic cell containing HCl; the Ag+ ions combine with Cl– ions as fast as they are formed at the silver surface.
- Standard Half-cell Potentials
Problem Example 1 Find the standard potential of the cell Cu(s) | Cu2+ || Cl– | AgCl(s) | Ag(s)and predict the direction of electron flow when the two electrodes are connected. Solution: The net reaction corresponding to this cell will be 2 Ag(s) +...
- Cell Description Conventions
In order to make it easier to describe a given electrochemical cell, a special symbolic notation has been adopted. In this notation the cell we described above would be Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s) There are several other conventions relating...
- Galvanic Cells
This arrangement is called a Galvanic cell. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The two...
- How To Balance Ionic Equations In Alkaline Medium?
(I) Standard Electrode Potential What information can we obtain from SEP? Sign – oxidation or reduction favoured Magnitude – the extent the reaction is favoured How to measure SEP? - connecting the redox couple (half cell) to SHE. - learn to draw...
- Electrochemistry : 10.1 Galvanic Cell
Electrochemistry Study of relationship between chemical change & electric work Oxidation Loss of electron by species accompanied byn an increase in oxidation number Ex: Reduction Gain electron by a species accompanied by a decrease number...
Electrochemistry
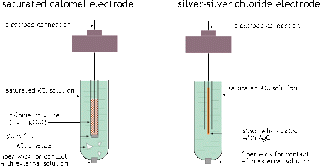
Reference Electrodes
In most electrochemical experiments our interest is concentrated on only one of the electrode reactions. Since all measurements must be on a complete cell involving two electrode systems, it is common practice to employ a reference electrode as the other half of the cell. The major requirements of a reference electrode are that it be easy to prepare and maintain, and that its potential be stable. The last requirement essentially means that the concentration of any ionic species involved in the electrode reaction must be held at a fixed value. The most common way of accomplishing this is to use an electrode reaction involving a saturated solution of an insoluble salt of the ion. One such system, the silver-silver chloride electrode has already been mentioned:
Ag | AgCl(s) | Cl–(aq) || ...
Ag(s) + Cl–(aq) →AgCl(s) + e–

The other common reference electrode is the calomel electrode; calomel is the common name for
mercury(I) chloride.Hg | Hg2+(aq) | KCl || ... Hg(l) + Cl– → ½ HgCl2(s) + e–
The potentials of both of these electrodes have been very accurately determined against the hydrogen electrode. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; the platinum surface has to be specially treated by preliminary electrolysis. Also, there is need for a supply of hydrogen gas which makes it somewhat cumbersome and hazardous.Some notes :
Make sure you thoroughly understand the following essential ideas which have been presented above. It is especially imortant that you know the precise meanings of all the highlighted terms in the context of this topic.
- A galvanic cell (sometimes more appropriately called a voltaic cell) consists of two half-cells joined by a salt bridge or some other path that allows ions to pass between the two sides in order to maintain electroneutrality.
- The conventional way of representing an electrochemical cell of any kind is to write the oxidation half reaction on the left and the reduction on the right. Thus for the reaction
Zn(s) + Cu2+ → Zn2+ + Cu(s)we writeZn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s)
in which the single vertical bars represent phase boundaries. The double bar denotes a liquid-liquid boundary which in laboratory cells consists of a salt bridge or in ion-permeable barrier. If the net cell reaction were written in reverse, the cell notation would become
Cu(s) | Cu2+(aq) || Zn 2+(aq) | Zn (s)
Remember: the Reduction process is always shown on the Right.
- The transfer of electrons between an electrode and the solution takes place by quantum-mechanical tunneling at the electrode surface. The energy required to displace water molecules from the hydration shell of an ion as it approaches the electrode surface constitutes an activation energy which can slow down the process. Even larger activation energies (and slower reactions) occur when a molecule such as O2 is formed or consumed.
- Standard Half-cell Potentials
Problem Example 1 Find the standard potential of the cell Cu(s) | Cu2+ || Cl– | AgCl(s) | Ag(s)and predict the direction of electron flow when the two electrodes are connected. Solution: The net reaction corresponding to this cell will be 2 Ag(s) +...
- Cell Description Conventions
In order to make it easier to describe a given electrochemical cell, a special symbolic notation has been adopted. In this notation the cell we described above would be Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s) There are several other conventions relating...
- Galvanic Cells
This arrangement is called a Galvanic cell. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The two...
- How To Balance Ionic Equations In Alkaline Medium?
(I) Standard Electrode Potential What information can we obtain from SEP? Sign – oxidation or reduction favoured Magnitude – the extent the reaction is favoured How to measure SEP? - connecting the redox couple (half cell) to SHE. - learn to draw...
- Electrochemistry : 10.1 Galvanic Cell
Electrochemistry Study of relationship between chemical change & electric work Oxidation Loss of electron by species accompanied byn an increase in oxidation number Ex: Reduction Gain electron by a species accompanied by a decrease number...
