Electrochemistry

Well aware of the fact that antroquinonol A lacked the kind of complexity that leaves people unsure of how it could ever be made, the goal from an academic perspective was to find a way to make antroquinonol that would be inherently interesting. In this spirit, Phil handed us a bottle of the dietary supplement Conezyme Q10 and said “why can’t we just reduce this into antroquinonol A?”.
So we quite literally isolated coenzyme Q10 from those pills and started playing around with this molecule. We had little hope this reaction could be done but I was a young second year and Phil is right in saying sometimes you just need to run the reaction. We came up with rationalizations about how the 4 consecutive oxygens on the benzene ring could reduce its aromaticity and found loosely related precedents but to no one’s surprise we were never able to get this reaction to work. Figure 1 of the paper quickly summarizes some of the things we tried but I’ll share a little more detail on two of the crazier ideas in the figure below.
 Solidly convinced that I was not going to be the one to come up with a way to reduce quinones into useful cyclohexane rings, we moved towards ring buildings strategies. Working with a talented postdoc, Dr. Eran Sella and a promising undergraduate, Garrett Saul, we were able to test out a good number of ideas on new ways to build substituted hydroxycyclohexenones. All were met eventually with failure but I’ll share with you a few of the molecules we made along the way.
Solidly convinced that I was not going to be the one to come up with a way to reduce quinones into useful cyclohexane rings, we moved towards ring buildings strategies. Working with a talented postdoc, Dr. Eran Sella and a promising undergraduate, Garrett Saul, we were able to test out a good number of ideas on new ways to build substituted hydroxycyclohexenones. All were met eventually with failure but I’ll share with you a few of the molecules we made along the way.

- Guest Post: A Philosophical Consideration Of Total Synthesis
This post comes from outside the Baran lab. A very talented undergraduate (Wade Miller) from the University of Pennsylvania sent us his paper from an assignment in philosophy class regarding the relevance of total synthesis in the modern era....
- Academia–industry Collaboration In The Route Optimization Of Taxadienone
In early 2012, we reported the gram-scale synthesis of a non-natural taxane, taxadienone, as well as that of a natural taxane, taxadiene. Now, chemists at Albany Molecular Research Inc. (AMRI) report a route optimization of taxadienone in none other than...
- Functionalized Olefin Cross-coupling
Figure 1. Functionalized olefin cross-coupling at a glance. The last paper of the year from the inner depths of our lab has made its way out earlier today. It’s the bigger brother of our reductive olefin coupling paper from January. In a nutshell, reductive...
- Diterpenoid-alkaloids Are So...fancy (you Already Know)
The second paper on our efforts toward diterpenes and related diterpenoid-alkaloids is out in JACS now. This is building off of work previously published in Angewante last year. The story of this project goes back more than 5 years. ...
- Column Chromatography....seems Silly Now.
Last month the team at Eisai responsible for the commercial scale synthesis of Halaven (easily the most complex drug molecule produced entirely by chemical synthesis) published a series of papers (theres three links there) detailing the development...
Electrochemistry
We made antroquinonol A then did things with it
Hello! I worked on this rather atypical paper and am here to tell you a little about how it progressed on the ground. As always, how the paper looks when it’s finished shares little resemblance to the journey. This is our only molecule that has its own youtube video.
A long long time ago in 2012, Bristol Myers Squibb (BMS) approached our group about working on the molecule Antroquinonol A due to interest in its anti-cancer and immunological properties. This molecule is not your typical “jungle-gym”-looking behemoth terpene that the Baran group usually pursues. Nor was it reported to have the kind of picomolar activity that makes medicinal chemists wake up in the morning. But there existed a litany of publications on the biological activity of this molecule and a company had deemed it worthy of significant financial investment, starting FDA clinical trials on the isolated natural product. This kind of molecule does not usually garner serious synthetic interest because it is not scary looking enough for an academic group but also a little too complicated to garner interest from many companies. However, at Scripps we have an interesting Academia-Industrycollaboration with BMS that I think seeks to fill this gap.
Well aware of the fact that antroquinonol A lacked the kind of complexity that leaves people unsure of how it could ever be made, the goal from an academic perspective was to find a way to make antroquinonol that would be inherently interesting. In this spirit, Phil handed us a bottle of the dietary supplement Conezyme Q10 and said “why can’t we just reduce this into antroquinonol A?”.
So we quite literally isolated coenzyme Q10 from those pills and started playing around with this molecule. We had little hope this reaction could be done but I was a young second year and Phil is right in saying sometimes you just need to run the reaction. We came up with rationalizations about how the 4 consecutive oxygens on the benzene ring could reduce its aromaticity and found loosely related precedents but to no one’s surprise we were never able to get this reaction to work. Figure 1 of the paper quickly summarizes some of the things we tried but I’ll share a little more detail on two of the crazier ideas in the figure below.
Funnily enough, the route that worked and is reported in the paper was one of the first things we proposed but was shelved to try more academically interesting ideas first. You can get enough details directly from the paper (or from other bloggers) so I won’t go through it again here. As you can imagine, even this less ambitious route was met with your typical disasters but after ~9 months we had a reliable way to make a lot of antroquinonol A. I will say that it was truly fun to get to work on a molecule small enough to try so many ideas and that brainstorming these wacky ideas with lab members will be one of my favorite memories of graduate school.
So as you can read in the paper (since its open access!), we sent off a vial full of this compound to BMS for testing, only to be surprised that their data did not match what was reported in the literature. I spent most of the next year trying to convince myself that I hadn’t screwed something up. The team at BMS proposed that a metabolite of antroquinonol may be the active component and did great work identifying a not previously reported structure. We synthesized enough to test and found that this molecule was also inactive. We made the opposite enantiomer just to be cautious (even though our optical rotations matched) and also found that to be inactive. It’s beyond my scope as a synthetic chemist to guess what is really going on here but from what I have read, it is not that uncommon of a problem.
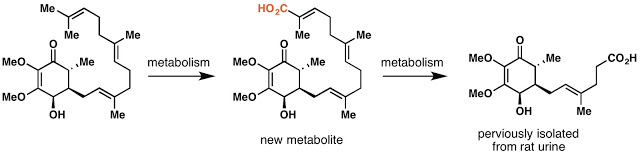
In the end I think this project was a good demonstration of why synthesis can still be relevant. Access to gram quantities of a pure, synthetically derived natural product has shed new light on a drug that is currently being tested on actual people. The mixing of interests from both industry and academia allowed us to work on a molecule of genuine importance but in an academically free way that was not just about producing the molecule as quickly as possible.
- Guest Post: A Philosophical Consideration Of Total Synthesis
This post comes from outside the Baran lab. A very talented undergraduate (Wade Miller) from the University of Pennsylvania sent us his paper from an assignment in philosophy class regarding the relevance of total synthesis in the modern era....
- Academia–industry Collaboration In The Route Optimization Of Taxadienone
In early 2012, we reported the gram-scale synthesis of a non-natural taxane, taxadienone, as well as that of a natural taxane, taxadiene. Now, chemists at Albany Molecular Research Inc. (AMRI) report a route optimization of taxadienone in none other than...
- Functionalized Olefin Cross-coupling
Figure 1. Functionalized olefin cross-coupling at a glance. The last paper of the year from the inner depths of our lab has made its way out earlier today. It’s the bigger brother of our reductive olefin coupling paper from January. In a nutshell, reductive...
- Diterpenoid-alkaloids Are So...fancy (you Already Know)
The second paper on our efforts toward diterpenes and related diterpenoid-alkaloids is out in JACS now. This is building off of work previously published in Angewante last year. The story of this project goes back more than 5 years. ...
- Column Chromatography....seems Silly Now.
Last month the team at Eisai responsible for the commercial scale synthesis of Halaven (easily the most complex drug molecule produced entirely by chemical synthesis) published a series of papers (theres three links there) detailing the development...
