Electrochemistry
An activity series is a list of substances ranked in order of relative reactivity. For example, magnesium metal can knock hydrogen ions out of solution, so it is considered more reactive than elemental hydrogen:
of metals below it to their elemental forms).
The activity series is a useful guide for predicting the products of metal displacement reactions. For example, placing a strip of zinc metal in a copper(II) sulfate solution will produce metallic copper and zinc sulfate, since zinc is above copper on the series. A strip of copper placed into a zinc sulfate solution will not produce an appreciable reaction, because copper is below zinc on the series and can't displace zinc ions from solution.
The series works well as long as the reactions being predicted occur at room temperature and in aqueous solution. It isn't difficult to find reactions that are at odds with the metal and nonmetal activity series under other conditions. There are other complications too. For example, aluminum would be expected to displace hydrogen from steam, but in fact it won't unless the aluminum oxide film on its surface is scrubbed off. Copper can't displace hydrogen from acids, but it does react with acids like nitric and sulfuric because they can act as oxidizing agents.
It might be expected that metals with lower ionization energies and lower electronegativities would be more active, since they would be expected to more easily lose electrons in a displacement reaction. But while ionization energy and electronegativity do affect a metal's ranking in the series, other factors have a strong and complex influence on relative activity. obscuring the relationship.
Activity series can be devised for nonmetals as well. Since nonmetallic elements tend to accept electrons in redox reactions, the nonmetal activity series is arranged so that the most powerful oxidizing agents are considered most active (whereas in the metal series, the most powerful reducing agents are the most active):
For example, the series predicts that Cl2 will displace Br- and I- from solution, because Cl2 appears above Br2 and I2:
- Galvanic Cells
This arrangement is called a Galvanic cell. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The two...
- O-level Chemistry Syllabus (2008)
1. Experimental Chemistry • Experimental design • Methods of purification and analysis • Identification of ions and gases 2. The Particulate Nature of Matter • Kinetic particle theory • Atomic structure • Structure and properties of materials...
- Electrochemistry
Learning Objectives(a) describe and explain redox processes in terms of electron transfer and/or of changes in oxidation number (oxidation state) (b) define the terms: (i) standard electrode (redox) potential (ii)...
- Electrochemistry : 10.3 : Electrolysis Cell
VOLTAN CELL VS ELECTRIOLYSIS CELL . Voltaic cell :use a spontaneous reaction to generate electric energy. Electrolysis :use electric energy to drive non- spontaneous energy. VOLTAIC CELL. ELECTROLYTICelectrons generate...
- Electrochemistry : 10.1 Galvanic Cell
Electrochemistry Study of relationship between chemical change & electric work Oxidation Loss of electron by species accompanied byn an increase in oxidation number Ex: Reduction Gain electron by a species accompanied by a decrease number...
Electrochemistry
What is an activity series, and how is it used?
An activity series is a list of substances ranked in order of relative reactivity. For example, magnesium metal can knock hydrogen ions out of solution, so it is considered more reactive than elemental hydrogen:
Mg(s) + 2 H+(aq)  H2(g) + Mg2+(aq)
H2(g) + Mg2+(aq)
Zinc can also displace hydrogen ions from solution:  H2(g) + Mg2+(aq)
H2(g) + Mg2+(aq) Zn(s) + 2 H+(aq)  H2(g) + Zn2+(aq)
H2(g) + Zn2+(aq)
so zinc is also more active than hydrogen. But magnesium metal can remove zinc ions from solution:  H2(g) + Zn2+(aq)
H2(g) + Zn2+(aq)Mg(s) + Zn2+(aq)  Zn(s) + Mg2+(aq)
Zn(s) + Mg2+(aq)
The reaction goes nearly to completion. Magnesium is more active than zinc, and the activity series including these elements would be Mg > Zn > H. The following activity series built up in a similar way. The most active metals are at the top of the table; the least active are at the bottom. Each metal is able to displace the elements below it from solution (or, using the language of electrochemistry, each metal can reduce the cations  Zn(s) + Mg2+(aq)
Zn(s) + Mg2+(aq)of metals below it to their elemental forms).
| displace H2 from water, steam, or acids | Li | 2 Li(s) + 2 H2O(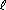 ) )  2 LiOH(aq) + H2(g) 2 LiOH(aq) + H2(g) |
| K | 2 K(s) + 2 H2O(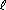 ) )  2 KOH(aq) + H2(g) 2 KOH(aq) + H2(g) | |
| Ca | Ca(s) + 2 H2O(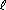 ) )  Ca(OH)2(s) + H2(g) Ca(OH)2(s) + H2(g) | |
| Na | 2 Na(s) + 2 H2O(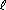 ) )  2 NaOH(aq) + H2(g) 2 NaOH(aq) + H2(g) | |
| displace H2 from steam or acids | Mg | Mg(s) + 2 H2O(g)  Mg(OH)2(s) + H2(g) Mg(OH)2(s) + H2(g) |
| Al | 2 Al(s) + 6 H2O(g)  2 Al(OH)3(s) + 3 H2(g) 2 Al(OH)3(s) + 3 H2(g) | |
| Mn | Mn(s) + 2 H2O(g)  Mn(OH)2(s) + H2(g) Mn(OH)2(s) + H2(g) | |
| Zn | Zn(s) + 2 H2O(g)  Zn(OH)2(s) + H2(g) Zn(OH)2(s) + H2(g) | |
| Fe | Fe(s) + 2 H2O(g)  Fe(OH)2(s) + H2(g) Fe(OH)2(s) + H2(g) | |
| displace H2 from acids only | Ni | Ni(s) + 2 H+(aq)  Ni2+(aq) + H2(g) Ni2+(aq) + H2(g) |
| Sn | Sn(s) + 2 H+(aq)  Sn2+(aq) + H2(g) Sn2+(aq) + H2(g) | |
| Pb | Pb(s) + 2 H+(aq)  Pb2+(aq) + H2(g) Pb2+(aq) + H2(g) | |
| H2 | ||
| can't displace H2 | Cu | |
| Ag | ||
| Pt | ||
| Au |
The activity series is a useful guide for predicting the products of metal displacement reactions. For example, placing a strip of zinc metal in a copper(II) sulfate solution will produce metallic copper and zinc sulfate, since zinc is above copper on the series. A strip of copper placed into a zinc sulfate solution will not produce an appreciable reaction, because copper is below zinc on the series and can't displace zinc ions from solution.
The series works well as long as the reactions being predicted occur at room temperature and in aqueous solution. It isn't difficult to find reactions that are at odds with the metal and nonmetal activity series under other conditions. There are other complications too. For example, aluminum would be expected to displace hydrogen from steam, but in fact it won't unless the aluminum oxide film on its surface is scrubbed off. Copper can't displace hydrogen from acids, but it does react with acids like nitric and sulfuric because they can act as oxidizing agents.
It might be expected that metals with lower ionization energies and lower electronegativities would be more active, since they would be expected to more easily lose electrons in a displacement reaction. But while ionization energy and electronegativity do affect a metal's ranking in the series, other factors have a strong and complex influence on relative activity. obscuring the relationship.
Activity series can be devised for nonmetals as well. Since nonmetallic elements tend to accept electrons in redox reactions, the nonmetal activity series is arranged so that the most powerful oxidizing agents are considered most active (whereas in the metal series, the most powerful reducing agents are the most active):
| F2 | strongest oxidizing agent |
| Cl2 | |
| O2 | |
| Br2 | |
| I2 | |
| S | |
| red P | weakest oxidizing agent |
Cl2(g) + 2 Br-(aq)  2 Cl-(aq) + Br2(
2 Cl-(aq) + Br2(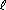 )
)
Cl2(g) + 2 I-(aq) 2 Cl-(aq) + I2(s)
2 Cl-(aq) + I2(s)
Br2(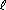 ) + 2 Cl-(aq)
) + 2 Cl-(aq)  no reaction
no reaction
I2(s) + 2 Cl-(aq) no reaction
no reaction
 2 Cl-(aq) + Br2(
2 Cl-(aq) + Br2(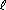 )
)Cl2(g) + 2 I-(aq)
 2 Cl-(aq) + I2(s)
2 Cl-(aq) + I2(s)Br2(
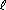 ) + 2 Cl-(aq)
) + 2 Cl-(aq)  no reaction
no reactionI2(s) + 2 Cl-(aq)
 no reaction
no reaction- Galvanic Cells
This arrangement is called a Galvanic cell. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The two...
- O-level Chemistry Syllabus (2008)
1. Experimental Chemistry • Experimental design • Methods of purification and analysis • Identification of ions and gases 2. The Particulate Nature of Matter • Kinetic particle theory • Atomic structure • Structure and properties of materials...
- Electrochemistry
Learning Objectives(a) describe and explain redox processes in terms of electron transfer and/or of changes in oxidation number (oxidation state) (b) define the terms: (i) standard electrode (redox) potential (ii)...
- Electrochemistry : 10.3 : Electrolysis Cell
VOLTAN CELL VS ELECTRIOLYSIS CELL . Voltaic cell :use a spontaneous reaction to generate electric energy. Electrolysis :use electric energy to drive non- spontaneous energy. VOLTAIC CELL. ELECTROLYTICelectrons generate...
- Electrochemistry : 10.1 Galvanic Cell
Electrochemistry Study of relationship between chemical change & electric work Oxidation Loss of electron by species accompanied byn an increase in oxidation number Ex: Reduction Gain electron by a species accompanied by a decrease number...
