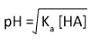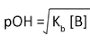Ionic Equilibria (Acid/ Base)
There are two broad aspects of this chapter:
- Acid/ Base Equilibria
- Solubility Equilibria
Acid/ Base Equilibria
Important Relations
- p = -log10
At 25°C:
- pH + pOH = pKw = 14
- pKa + pKb = 14
- [H+][OH–] = 10-14
- Ka x Kb = Kw = 10-14
Approach:
Determine what is present in solution: Acid/ Base/ Salt/ Buffer
Apply the corresponding equations:
Acid/ Base
| Formula | Example | |
| Strong Acid | [H+] = [HA] pH = -log [HA] | pH of 0.100 moldm–3 HCl
pH = -log [0.100] = 1.0 |
| Strong Base | [OH–] = [B] pOH = -log [B] | pH of 0.100 moldm–3 NaOH
pOH = -log [0.100] = 1.0 pH = 14.0 - 1.0 = 13.0 |
| Weak Acid |
pH = -log [H+] | pH of 0.100 moldm–3 CH3COOH (Ka = 1.8 x 10–5 moldm–3)
[H+] = [(1.8 x 10–5)(0.100)]1/2 = 1.34 x 10–3 mol dm–3 pH = -log [1.34 x 10–3] = 2.9 |
| Weak Base |
pOH = -log [OH–] | pH of 0.100 moldm–3 NH3 (Kb = 1.8 x 10–5 moldm–3)
[OH–] = [(1.8 x 10–5)(0.100)]1/2 = 1.34 x 10–3 mol dm–3 pOH = -log [1.34 x 10–3] = 2.9 pH = 14 - 2.9 = 11.1 |
Salt
| Nature of Salt | Formed from | pH |
| Neutral | strong acid + strong base | = 7 |
| Acidic | strong acid + weak base | Treat as weak acid; use weak acid formula |
| Basic | weak acid + strong base | Treat as weak base; use weak base formula |
In calculating the pH of a salt solution, there are two common challenges:
1. Ka/ Kb of the salt (conjugate acid/ conjugate base) is not given.
Using Ka x Kb = Kw, find Ka/ Kb of the ion from Kb/ Ka of the parent base/ acid given.
2. finding concentration of salt.
[salt] = n (limiting reagent)/ Vtotal [Vtotal = Vacid + Vbase]
Buffer
| Type | Formed from | pH |
| Acidic | weak acid + conjugate base | < 7 pH = pKa + log [salt]/[acid] |
| Basic | weak base + conjugate acid | > 7 pOH = pKb + log [salt]/[base] |
How to calculate pH of a buffer on adding small amounts of H+ and OH–?
- Determine which species is reacted and which is formed.
- Calculate new amounts (in moles)
- Substitute into buffer equation
Note:
- When using H-H equation, just calculate no. of moles of salt and acid/base since total volume is the same and cancels out.
- Look out for [salt] = [acid] (max. buffering capacity) → pH = pKa (same for basic buffer)
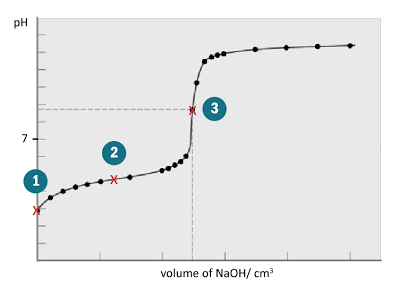
Titration Curve
- There are three important points on the titration curve:
Example: Titration of weak acid against strong base
|
| Titration curve | What to observe | What can be found |
| 1 | Initial point | pH | [H+] due to dissociation of acid |
| 2 | Equivalence point | volume of base pH | Given [acid], [base] can be found Given [base], [acid] can be found type of salt/ type of titration |
| 3 | Half equivalence point | pH | pKa of acid |
- Calculate pH at various points in titration curve
Strategy
- Determine what is in solution: Acid/ Base/ Salt/ Buffer
- Use the relevant equations.
Example: Titration of weak acid against strong base:
| Titration curve | What is in solution |
| Initial point | Weak acid |
| Between initial point to equivalence point | Acidic buffer |
| Equivalence point | Basic Salt |
| Beyond equivalence point | Strong base |
- Determine Structure Of Undigested Protein
← Back to Amino Acids & Proteins Digestion of proteins can be carried out using 2 methods: Acid-Base Hydrolysis Enzyme Cleavage Non-selective Overlapping fragments Selective No...
- Titration
← Back to AMS Normal Titration See general approach for chemical calculations. Example: 25.00 cm3 of 0.100 moldm–3 Na2CO3 solution was titrated against 0.200 moldm–3 HCl. Determine the volume of HCl required...
- H2 Chemistry Syllabus (2008)
PHYSICAL CHEMISTRY 1. ATOMS, MOLECULES AND STOICHIOMETRY • Relative masses of atoms and molecules • The mole, the Avogadro constant • The calculation of empirical and molecular formulae • Reacting masses and volumes (of solutions and gases) 2....
- H1 Chemistry Syllabus (2008)
PHYSICAL CHEMISTRY 1. ATOMS, MOLECULES AND STOICHIOMETRY • Relative masses of atoms and molecules • The mole, the Avogadro constant • The calculation of empirical and molecular formulae • Reacting masses and volumes (of solutions and gases) 2....
- Thermochemistry : 9.1 Concept Of Enthalpy
Heat-The energy transferred between a system and surrounding. System-Part of universe whose change we are going to observe. Surrounding-The rest of the universe outside the system. Exothermic-Gives off heat.-From the system to the surroundings.-Ex : 2H2(g)...