Titration
← Back to AMS
Normal Titration
See general approach for chemical calculations.
| Example: 25.00 cm3 of 0.100 moldm–3 Na2CO3 solution was titrated against 0.200 moldm–3 HCl. Determine the volume of HCl required to reach equivalence point.
Na2CO3 + 2 HCl → 2 NaCl + CO2 + H2O
Step 1: Find the amount of Na2CO3. n(Na2CO3) = 25.00/1000 x 0.100 = 0.0025 mol
Step 2: Use reacting ratio to find amount of HCl required. Na2CO3 : HCl = 1 : 2 n(HCl) = 2 x 0.0025 = 0.0050 mol V(HCl) = 0.025 dm3 = 25.0 cm3 |
Sequential Titration
A + B → C --- (1)
C + D → E --- (2)
What is happening:
A is reacted with B to produce C.
C is then further reacted with D.
Objective: Given amount of D, find amount of A.
Approach:
- Find amount of D.
- Using the reacting ratio for rxn (2), find amount of C.
- Using the reacting ratio for rxn (1), find amount of A.
Shortcut:
Find the reacting ratio between A and D, and use it to find amount of A directly.
Back Titration
A + excess B → I --- (1)
remaining B + C → P --- (2)
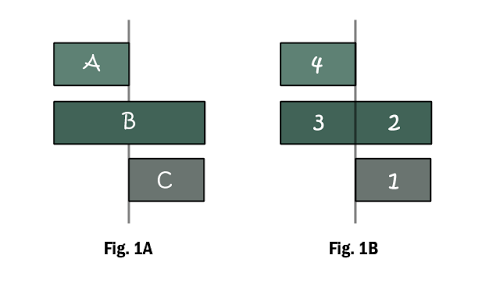
What is happening: (Fig. 1A)
A is reacted with excess B.
Remaining B is then reacted with C.
Objective: Given amount of C and total amount of B used, find amount of A.
Approach: (Fig. 1B)
- Identify A, B and C. Find amount of C.
- Use reacting ratio in rxn (2), find the amount of B remaining.
- From the total amount of B used in rxn (1), find the amount of B that had reacted with A.
- Use reacting ratio in rxn (1), find the amount of A present.
| Example: A sample of Na2CO3 (106 g/mol) was added to 20 cm3 of 0.500 moldm–3 HCl. The resultant solution was then titrated with 0.100 moldm–3 NaOH. 22.00 cm3 of NaOH was used. Determine the mass of Na2CO3 added.
Na2CO3 + 2 HCl → 2 NaCl + CO2 + H2O ----- (1) HCl + NaOH → NaCl + H2O ----- (2) A: Na2CO3; B: HCl; C: NaOH
Step 1: Find the amount of C n(NaOH) = 22.00/1000 x 0.1 = 0.0022 mol
Step 2: Use reacting ratio in rxn (2), find the amount of B remaining. HCl : NaOH = 1 : 1 n(HCl) remaining = 0.0022 mol
Step 3: From the total amount of B used in rxn (1), find the amount of B that had reacted with A. n(HCl) total = 20/1000 x 0.5 = 0.01 mol n(HCl) reacted with Na2CO3 = 0.01 - 0.0022 = 0.0078 mol
Step 4: Use reacting ratio in rxn (1), find the amount of A present. Na2CO3 : HCl = 1 : 2 n(Na2CO3) = 1/2 x 0.0078 = 0.0039 mol m(Na2CO3) = n(Na2CO3) x Mr(Na2CO3) = 0.0039 x 106 = 0.413 g |
- Faraday Law
FARADAY LAWAmount of substance produce of each electrode is directly proportional to quantity of charge flowing through the cell Also called Faraday's First Law of electrolysis. CALCULATING USING FARADAY'S LAWMain stepsBalance half reaction to...
- Combustion Analysis
← Back to AMS Combustion analysis questions commonly fall into 3 types: Find CxHy given VCO2, VO2, VCxHy Find CxHy given ∆V, VCO2, VCxHy Find CxHyOz given mCO2, mH2O, mCxHy 1. Find CxHy given VCO2, VO2, VCxHy CxHy...
- Dilution Versus Sampling
← Back to AMS Analogy Imagine the scenario where a sachet of milo powder is dissolved in 100 cm3 of water. Dilution – Another 100 cm3 of water is added to the solution. Is the amount of milo powder the same in 1 & 2? Is the sweetness...
- Redox Titrations (unknown Oxidation No.)
← Back to Electrochemistry Determine the reacting ratio between the reactants. Construct the following table: Rxt A Rxt B Reacting ratio P ...
- Ionic Equilibria (acid/ Base)
There are two broad aspects of this chapter: Acid/ Base Equilibria Solubility Equilibria Acid/ Base Equilibria Important Relations p = -log10 At 25°C: pH + pOH = pKw = 14 pKa + pKb = 14 [H+][OH–] = 10-14 Ka x Kb = Kw = 10-14 Approach:...
