Electrochemistry
- Let Recruiting Season Begin!
Well, it's that time of year again. You know, the one where I look out my window at the Torrey Pines Golf Course and the ocean, think about going to the zoo in a t-shirt and shorts, and then walk out to my car that is not stuck in the snow. But really,...
- Substituent Effects
← Back to Arenes A substituent on a benzene ring can have 2 effects: activate or deactivate the ring i.e. cause the substituted benzene to be more reactive or less reactive than benzene direct the position of subsequent substitution i.e. 2,4-directing...
- Determine Products Of Side Chain Oxidation
← Back to Arenes Benzylic C (C next to the benzene ring) is oxidized to benzoic acid (or benzoate under alkaline conditions) All other C in the side chain are oxidized to CO2 For side chain oxidation to occur,...
- Determine No. Of Stereoisomers
← Back to Intro to Org Chem no. of stereoisomers = 2n n = no. of stereogenic centers i.e. chiral C + C=C or cyclic rings that can exhibit cis/trans Students make mistakes when they blindly count the total number of C=C...
- Determine No. Of Structural Isomers
← Back to Intro to Org Chem [Bad News] There is no mathematical formula available to calculate the number of structural isomers. You have to draw out all possible structures. [Good News] There is a general approach for that. ...
Electrochemistry
On Benzene
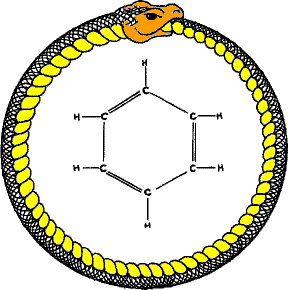 |
| Figure 1. Snaaaaaakeeeee, a snaaaaaakeeeee! |
Ahh benzene! Europeans fear it, I love the smell of it, and it can look like a snake (Figure 1)! What isn't there to love? Not to mention its qualities as a solvent, and its utility in the azeotropic removal of water from ethanol. But what I'm here to discuss today is how we draw benzene (dude, lets be honest, the Armstrong benzene is fantastic). This came up over coffee a few years ago and I decided to formalize it a little bit. If you want to play along, go ahead and draw a benzene yourself, don't think too hard, but pay attention to how you draw it. Twenty-five graduate student and postdoctoral chemists were surveyed for this blog post and the results were compiled and analyzed for your pleasure (after the jump).
Read more »
- Let Recruiting Season Begin!
Well, it's that time of year again. You know, the one where I look out my window at the Torrey Pines Golf Course and the ocean, think about going to the zoo in a t-shirt and shorts, and then walk out to my car that is not stuck in the snow. But really,...
- Substituent Effects
← Back to Arenes A substituent on a benzene ring can have 2 effects: activate or deactivate the ring i.e. cause the substituted benzene to be more reactive or less reactive than benzene direct the position of subsequent substitution i.e. 2,4-directing...
- Determine Products Of Side Chain Oxidation
← Back to Arenes Benzylic C (C next to the benzene ring) is oxidized to benzoic acid (or benzoate under alkaline conditions) All other C in the side chain are oxidized to CO2 For side chain oxidation to occur,...
- Determine No. Of Stereoisomers
← Back to Intro to Org Chem no. of stereoisomers = 2n n = no. of stereogenic centers i.e. chiral C + C=C or cyclic rings that can exhibit cis/trans Students make mistakes when they blindly count the total number of C=C...
- Determine No. Of Structural Isomers
← Back to Intro to Org Chem [Bad News] There is no mathematical formula available to calculate the number of structural isomers. You have to draw out all possible structures. [Good News] There is a general approach for that. ...
