Electrochemistry
- Making C–c Bonds With The Simplicity Of Making Amide Bonds
Despite all of the wonderful advances in organic synthesis, amide-bond formation is still the most widely used reaction among those making medicines. This truth has led some to declare their embarrassment and depression. Medicinal...
- Diversinate Update
Since our first report in 2011 on the trifluoromethylation of heterocycles, sulfinate chemistry has been demonstrated to be a useful tool for drug discovery. The lab extended this chemistry beyond trifluoromethyl radicals to a whole host of...
- The Difluoroethylator?
Over the past few years our laboratory has become interested in the radical functionalization of heterocycles. This started with arylboronic acids as radical precursors but recently developed into alkylsulfinate chemistry. The latest addition to our list...
- Alkanes (overview)
Structure & Bonding saturated: all C atoms are bonded to 4 atoms all C atom are sp3 hybridised non-polar simple molecular structures consisting of alkane molecules held together by weak VDW forces ...
- Writing Free Radical Substitution Mechanism
← Back to Alkanes When writing mechanisms: It is a good practice to write the name of the mechanism whether or not the question asks for it. You just need to show the series of equations/ arrows. There is no need for descriptions i.e. the...
Electrochemistry
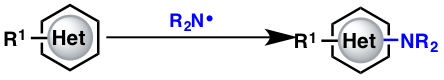
Playing with radicals
Aromatic C–H amination has received a lot of attention over the last few years, and our efforts began late 2012. The goal was simple: create a nitrogen-centered radical and trap it with a heterocycle.

Read more »
- Making C–c Bonds With The Simplicity Of Making Amide Bonds
Despite all of the wonderful advances in organic synthesis, amide-bond formation is still the most widely used reaction among those making medicines. This truth has led some to declare their embarrassment and depression. Medicinal...
- Diversinate Update
Since our first report in 2011 on the trifluoromethylation of heterocycles, sulfinate chemistry has been demonstrated to be a useful tool for drug discovery. The lab extended this chemistry beyond trifluoromethyl radicals to a whole host of...
- The Difluoroethylator?
Over the past few years our laboratory has become interested in the radical functionalization of heterocycles. This started with arylboronic acids as radical precursors but recently developed into alkylsulfinate chemistry. The latest addition to our list...
- Alkanes (overview)
Structure & Bonding saturated: all C atoms are bonded to 4 atoms all C atom are sp3 hybridised non-polar simple molecular structures consisting of alkane molecules held together by weak VDW forces ...
- Writing Free Radical Substitution Mechanism
← Back to Alkanes When writing mechanisms: It is a good practice to write the name of the mechanism whether or not the question asks for it. You just need to show the series of equations/ arrows. There is no need for descriptions i.e. the...
