Electrochemistry
The preparation is slightly different from that of the original paper. (Tetrahedron, 1996, 52, 2377–2384.)
Methyl(trifluoromethyl)dioxirane (TFDO): A 500-mL, three-necked, round-bottomed flask was equipped with a large stir bar and a condenser that was attached to a 25 or 50-mL receiving flask, cooled at -78 °C (acetone bath with dry ice). A hose connector must exist between the condenser and the flask to allow for pressure release/condensation (see picture 1).

- Reference Electrodes
In most electrochemical experiments our interest is concentrated on only one of the electrode reactions. Since all measurements must be on a complete cell involving two electrode systems, it is common practice to employ a reference...
- Ppm/ Ppb
← Back to AMS Parts per million, ppm is just another unit of concentration (like mol dm–3, g dm–3 etc) i.e. 1 part solute in 1 million parts solvent. For solutions, it simply means the grams of solute per million grams of solution....
- Dilution Versus Sampling
← Back to AMS Analogy Imagine the scenario where a sachet of milo powder is dissolved in 100 cm3 of water. Dilution – Another 100 cm3 of water is added to the solution. Is the amount of milo powder the same in 1 & 2? Is the sweetness...
- Ionic Equilibria (acid/ Base)
There are two broad aspects of this chapter: Acid/ Base Equilibria Solubility Equilibria Acid/ Base Equilibria Important Relations p = -log10 At 25°C: pH + pOH = pKw = 14 pKa + pKb = 14 [H+][OH–] = 10-14 Ka x Kb = Kw = 10-14 Approach:...
- Introduction To Organic Chemistry : 12.5 Organic Reaction
TYPE OF CLEAVAGE : 2 type of bond cleavage:- Hemolytic cleavageHeterolytic cleavage Hemolytic cleavage The breaking of a single (two-electron) bond in which one electron remains on each of the atoms. Also known as free-radical reaction; homolysis. ...
Electrochemistry
TFDO Synthesis Procedure
TFDO aka methyl(trifluoromethyl)dioxirane is a very powerful reagent for the C–H oxidation of unactivated alkanes. Unfortunately, it is not commercially available and consequently needs to be prepared. Due to the volatility of TFDO, its preparation is sometimes seen as tedious if not finicky, but with the correct set-up and directions, we make TFDO without fail! It’s actually a sort of rite of passage for people working on C–H oxidation in the lab!
The preparation is slightly different from that of the original paper. (Tetrahedron, 1996, 52, 2377–2384.)
Methyl(trifluoromethyl)dioxirane (TFDO): A 500-mL, three-necked, round-bottomed flask was equipped with a large stir bar and a condenser that was attached to a 25 or 50-mL receiving flask, cooled at -78 °C (acetone bath with dry ice). A hose connector must exist between the condenser and the flask to allow for pressure release/condensation (see picture 1).

The cooled (ice-water bath) three-necked flask was charged with a slurry of NaHCO3 (26.0 g) in water (26 mL), then solid Oxone® (48 g) was added to the vigorously stirred slurry of NaHCO3 (see Note below, addition takes place over 1–2 min). A lot of CO2 gas evolves during this process (see picture 2).

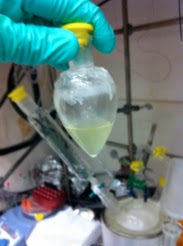
After 2 min, the pre-cooled (-20 °C, freezer temperature) liquid addition funnel was placed on the three-necked flask and was quickly charged with trifluoroacetone (24.0 mL). Then, the trifluoroacetone was added within ca. 10 s. After a few seconds, the pale yellow solution of the methyl(trifluoromethyl)dioxirane (TFDO) in trifluoroacetone was collected in the cooled (-78 °C) receiving flask (see pictures 3 and 4).

After 20 min, the receiving flask was tightly closed with a plastic stopper (yellow cap). The TFDO yield was 2.0 ± 0.5% (relative to trifluoroacetone) and its concentration was determined iodometrically (0.5 mL H20, 1.5 mL glacial acetic acid, 0.25 mL saturated KI solution; addition of 0.100 mL of the TFDO solution at –78 °C; titration with a freshly standardized 0.05 N Na2S203 solution). The concentration of TFDO in trifluoroacetone ranged typically from 0.4 to 0.6 M and the volume from 4 to 7 mL. Then, the receiving flask was wrapped with aluminum foil to protect the TFDO from light and stored at –80 °C (this reagent can be stored for several months without a drop in concentration).
Important things!!
Good Luck!
Quentin & Guillaume

After 2 min, the pre-cooled (-20 °C, freezer temperature) liquid addition funnel was placed on the three-necked flask and was quickly charged with trifluoroacetone (24.0 mL). Then, the trifluoroacetone was added within ca. 10 s. After a few seconds, the pale yellow solution of the methyl(trifluoromethyl)dioxirane (TFDO) in trifluoroacetone was collected in the cooled (-78 °C) receiving flask (see pictures 3 and 4).


Important things!!
- Wash all of your glassware with a solution of EDTA (c=0.1M) before the reaction to avoid any trace metal contaminants.
- You need a very good stirring plate with a big magnetic stir bar
- You can add the Oxone® in 5 g portions
- Make sure the system is all closed before the condenser; after the condenser, there should be an open hose connector to release pressure and condense TFDO.
- The quality of the Oxone® is essential (the one from Aldrich is very good) and you have to crush it into a fine powder before use.
- The quality of the trifluoroacetone is also essential (also from Aldrich)
- Use Millipore water
Good Luck!
Quentin & Guillaume
- Reference Electrodes
In most electrochemical experiments our interest is concentrated on only one of the electrode reactions. Since all measurements must be on a complete cell involving two electrode systems, it is common practice to employ a reference...
- Ppm/ Ppb
← Back to AMS Parts per million, ppm is just another unit of concentration (like mol dm–3, g dm–3 etc) i.e. 1 part solute in 1 million parts solvent. For solutions, it simply means the grams of solute per million grams of solution....
- Dilution Versus Sampling
← Back to AMS Analogy Imagine the scenario where a sachet of milo powder is dissolved in 100 cm3 of water. Dilution – Another 100 cm3 of water is added to the solution. Is the amount of milo powder the same in 1 & 2? Is the sweetness...
- Ionic Equilibria (acid/ Base)
There are two broad aspects of this chapter: Acid/ Base Equilibria Solubility Equilibria Acid/ Base Equilibria Important Relations p = -log10 At 25°C: pH + pOH = pKw = 14 pKa + pKb = 14 [H+][OH–] = 10-14 Ka x Kb = Kw = 10-14 Approach:...
- Introduction To Organic Chemistry : 12.5 Organic Reaction
TYPE OF CLEAVAGE : 2 type of bond cleavage:- Hemolytic cleavageHeterolytic cleavage Hemolytic cleavage The breaking of a single (two-electron) bond in which one electron remains on each of the atoms. Also known as free-radical reaction; homolysis. ...
